Chopra C, Filipowska L, Wang S. Evaluating the impact of remdesivir on COVID-19 patient outcomes: an inpatient county hospital analysis. HPHR. 2021; 27.
DOI:10.54111/0001/aa1
We performed a retrospective chart review on COVID-19 patients treated at Arrowhead Regional Medical Center (ARMC) in Colton, California from January through October 2020. Two outcomes were measured: time to recovery and mortality outcome. Models were subsequently generated to investigate the role of remdesivir on these patient outcomes.
This study compares data on the Top 20 MSAs with the highest cumulative COVID-19 case rate at two timepoints (October 13, 2020 and January 1, 2021). The means of CDC’s Social Vulnerability Index (SVI) variables for the highest risk MSAs were compared with the Welch Two Sample t-test to the means of the SVI variables for the rest of the U.S.
While initial research indicated that remdesivir decreased time to recovery, we found no such appreciable decrease in our study population. Our data corroborates existing studies on the lack of remdesivir’s impact on mortality. When controlling for days from symptom onset to administration of this medication, these outcomes do not change.
Our data demonstrate that remdesivir does not significantly alter time to recovery or mortality in COVID-19 patients. While the National Institute of Health (NIH) has sanctioned remdesivir’s use for certain COVID-19 populations, we do not observe any appreciable effects of this implementation at our county hospital. Increasing sample size and investigating inclusion criteria may help elucidate this observed lack of effect.
Since the World Health Organization (WHO) first announced COVID-19 as a global pandemic on March 11th, 2020, scientists have worked to develop evidence-based methods for effectively minimizing spread and treating those infected.1 Several drugs have received attention in the public sphere for their potential benefit in treating COVID-19, including hydroxychloroquine, convalescent plasma, tocilizumab, dexamethasone, and more recently, remdesivir.2,3,4,5 Remdesivir is a nucleotide analogue; its mechanism of action is preventing RNA subunit addition for viral replication.6 Remdesivir gained worldwide attention following the publication of trial results supporting its proposed efficacy as a treatment for COVID-19. The Adaptive COVID-19 Treatment Trial [ACTT-1] was a multinational, randomized, placebo-controlled trial conducted from February – April 2020 that demonstrated that individuals diagnosed with COVID-19 who were subsequently treated with remdesivir experienced a significantly shorter time to recovery.6 Based on the outcome of this research, the National Institute of Health (NIH) released guidelines in July of 2020 stating that all patients with COVID-19 who require noninvasive supplemental oxygen should be treated with 5 days of remdesivir to minimize hospital stay.7 Despite these NIH guidelines, research on the efficacy of remdesivir in treating COVID-19 has yielded mixed results.8,9,10
Prior to the ACTT-1 study, NIH standard of care guidelines for treatment of COVID-19 infection included a 10-day course of the corticosteroid dexamethasone for patients on supplemental oxygen. This standard of care was established based on the results of the RECOVERY trial, which demonstrated that among patients on mechanical ventilation or supplemental oxygen with COVID-19, those who received a 10-day course of dexamethasone experienced a statistically significant decrease in 28-day mortality.9
Given the results of the RECOVERY and ACTT-1 studies, we hypothesize that patients diagnosed with COVID-19 and treated with both remdesivir and dexamethasone will have a shorter time to recovery than those treated with dexamethasone alone. We also hypothesize that when controlling for dexamethasone, remdesivir will not have a significant impact on patient mortality.
Patient records were collected from the MediTech electronic medical database at Arrowhead Regional Medical Center (ARMC) in Colton, California. ARMC is a 456-bed hospital at the center of San Bernardino County’s public health care system, providing emergency, primary, and specialty care to 48,700 people. ARMC sees more than 275,000 visits annually in both inpatient and outpatient settings and is designated as a Level II Trauma Hospital.11
The records for 2,301 patients diagnosed with COVID-19 (ICD-10 code U07.1) were extracted for analysis. The date range for diagnosed patients was 01/01/2020 to 10/27/2020.
In accordance with the NIH recommendations released in July 2020, remdesivir was given to the above patients after they met the following additional criteria:
Because patients at ARMC were chosen to receive remdesivir if they were already being treated with dexamethasone, only patients treated with dexamethasone were included in the study. This filter removed the potential confounding effect of dexamethasone when comparing patients treated with both remdesivir and dexamethasone against patients who received neither drug. Similarly, as per NIH guidelines, the recommended course of remdesivir is 5 days6 and therefore, any patients who did not complete all 5 days of their prescribed remdesivir were excluded.6 Pregnant patients, prisoners, and patients under the age of 18 were also excluded.
Once a list of patients filtered by ICD-10 code was generated, the data was subsequently filtered to exclude patients with an inpatient treatment course of less than 10 days, as these patients were presumed to either 1) not require dexamethasone therapy, or 2) not complete the entire dexamethasone course inpatient. While some patients were prescribed dexamethasone to take orally at home, guaranteeing adherence to this pharmacotherapy was not possible and those patients were thus excluded due to potential noncompliance.
Patients were subsequently categorized into one of two cohorts:
In addition to classification of patients based on pharmacotherapy for COVID-19, a manual chart review was conducted to collect data on several other clinical descriptors among both groups (Table 1). Outcome variables were also collected and included mortality, and time to recovery (measured as total hospital length of stay).
Models were generated using Statistical Package for Social Sciences (SPSS) Version 26 software to observe the relationship between pharmacotherapy and patient outcome, which was evaluated using two criteria. The first outcome criterion was whether the patient expired. The second outcome criterion was time to recovery, which was recorded as total length of hospital stay.
Univariate analysis was conducted to summarize admissions, patient demographics (age and sex), oxygen status, comorbidities (diabetes, hypertension, COPD, obesity), time to recovery (total length of stay), mortality outcome (death vs. no death), and pharmacotherapy (dexamethasone, remdesivir). Comparative analysis was carried out to assess differences between pharmacotherapy and patient outcome (total length of stay, mortality).
These variables were entered into a logistic regression model to determine their ability to predict patient outcomes (death). No significant predictors were identified. A linear regression model to determine the effect on length of stay for patients receiving dexamethasone or remdesivir was also not significant. All data analysis was conducted use IBM SPSS Statistics 26.0 (Armonk, New York).
In total, 112 patient records were included in our analysis. 71 (63%) of these cases were male, and the average age of these patients was 60±13 years (Figure 1). 42 (37.5%) received a full 10-day course of dexamethasone only, and 70 (62.5%) received a full course of dexamethasone and a full 5-day course of remdesivir (Table 1).
Oxygen delivery method at the beginning of dexamethasone treatment was analyzed: the most common starting oxygen level for both dexamethasone-only patients remdesivir-treated patients was low flow nasal canula. Patients treated with dexamethasone only were significantly more likely to be on low-flow nasal cannula at the onset of treatment than those treated with remdesivir, and conversely, remdesivir-treated patients were significantly more likely to be on HiFlo cannula (Table 1).
Several comorbidities were also analyzed, and of the 112 patients, 62% had diabetes, 52% had hypertension, 47% were obese, and 7% were previously diagnosed with chronic obstructive pulmonary disease. Patients who subsequently received remdesivir were more likely to be diabetic, hypertensive, and obese than patients who only received dexamethasone. Of these parameters, patients who received remdesivir were significantly more likely to be diabetic than those who did not receive remdesivir. However, remdesivir-treated patients also trended toward higher rates of hypertension and obesity (Table 1).
Average time to recovery for patients treated with dexamethasone alone was shorter than that of those treated with dexamethasone and remdesivir. This observed difference was not statistically significant, and this did not change when controlling for number of days from symptom onset to treatment (Table 2).
The relative risk of death in COVID-19 patients treated with dexamethasone plus remdesivir as opposed to dexamethasone alone is 1.841 (95% CI = 0.863 – 3.93). However, given the confidence interval, we conclude that the observed relative risk is not statistically significant. Taken together, these statistics suggest that remdesivir use is not predictive of mortality in COVID-19 patients.
The overall mortality rate was 27%, while the treatment-specific mortality rate for the dexamethasone-only cohort was 19% and 31% in the remdesivir cohort. Of note, the average time from symptom onset to initiation of treatment was slightly lower for remdesivir-treated patients (5.3 ± 3.3 days) than those treated only with dexamethasone (7.1 ± 5.7 days) (Table 1). Additionally, time to death was shorter in patients treated with remdesivir than in those treated with dexamethasone only (Table 2).
The patients who expired during their COVID-19 hospitalization were more likely to be older, have higher oxygen requirements at the onset of their pharmacotherapy, and were more likely to be hypertensive than those who survived (Table 3). Of the patients who expired, patients who received remdesivir were younger, had shorter periods between symptom onset and treatment, had higher oxygen requirements at treatment onset, but were less likely to have diabetes, hypertension, obesity, or COPD (Table 2).
The results of this study are interesting to consider in the setting of the global search for an effective and proven pharmacotherapy for COVID-19.2 While our sample size is small, participants were selected carefully for drug therapy and compliance.
The results of our study fail to support our hypothesis that remdesivir is associated with a statistically significant decrease in time to recovery as measured by total length of hospital stay. In fact, patients treated with remdesivir trended to a longer time to recovery on average than those treated with dexamethasone alone (16.4 ± 10.7 days to recovery in dexamethasone patients, as opposed to 19.1 ± 12.5 days in patients treated with dexamethasone and remdesivir). This result may be explained by the fact that the patients receiving remdesivir were already more acutely ill at the onset of treatment when compared to those treated only with dexamethasone. This is evidenced by the generally observed sequence in which patients are administered remdesivir after already staring a course of dexamethasone. Time to treatment or a generally more severe infectious course may explain why, despite the addition of remdesivir, time to recovery was longer in the remdesivir-treated patients. This observation is further supported by the fact that oxygen level, which is commonly used as a measure of the patient’s clinical status, reflected a higher acuity among the remdesivir treatment group, with 40% of remdesivir patients receiving high-flow oxygen, as opposed to 14% of dexamethasone only patients receiving high-flow oxygen at the start of therapy. Of note, when controlling for days from symptom onset to dexamethasone treatment, patient outcomes did not change. This is important to consider, as it suggests that treating symptoms sooner may not yield a different clinical outcome as previously suggested.
Our study results are congruent with the current literature: remdesivir does not demonstrate a mortality benefit for COVID-19 patients.6,10,12 Like with time to recovery in patients who survived, this result may be explained by a potentially higher average-acuity state of the remdesivir-treated patients.
A limitation to this study is our sample size, which may have impacted our ability to achieve statistical significance. Nonetheless, it appears via qualitative analysis that the patients treated with remdesivir do not demonstrate better outcomes as predicted by the NIH guidelines. These considerations are valuable in a county hospital setting, as they demonstrate that clinically significant differences in outcomes may not be grossly observed on the frontlines despite a difference documented in a much larger study.
We suggest caution when inferring these results to nationwide trends due to the small sample size and potential limitation of increased severity of disease among remdesivir-treated patients. Future studies of interest may include exploring five vs. ten-day courses of remdesivir, as well as the drug’s impact on patients receiving mechanical ventilation or extracorporeal membrane oxygenation (ECMO).13
While remdesivir continues to be a supported drug therapy for select COVID-19 patients, our data highlights that in a smaller population in a county hospital setting, such differences in time to recovery may not be readily observed. We therefore recommend mediating expectations when prescribing remdesivir to enhance or change clinical outcome.
These results reinforce a need for further investigations into effective pharmacotherapies for COVID-19, as well as vaccination options, as remdesivir does not appear to provide the outcomes needed to overcome the pandemic as new cases continue to surge in the United States and across the globe.14,15
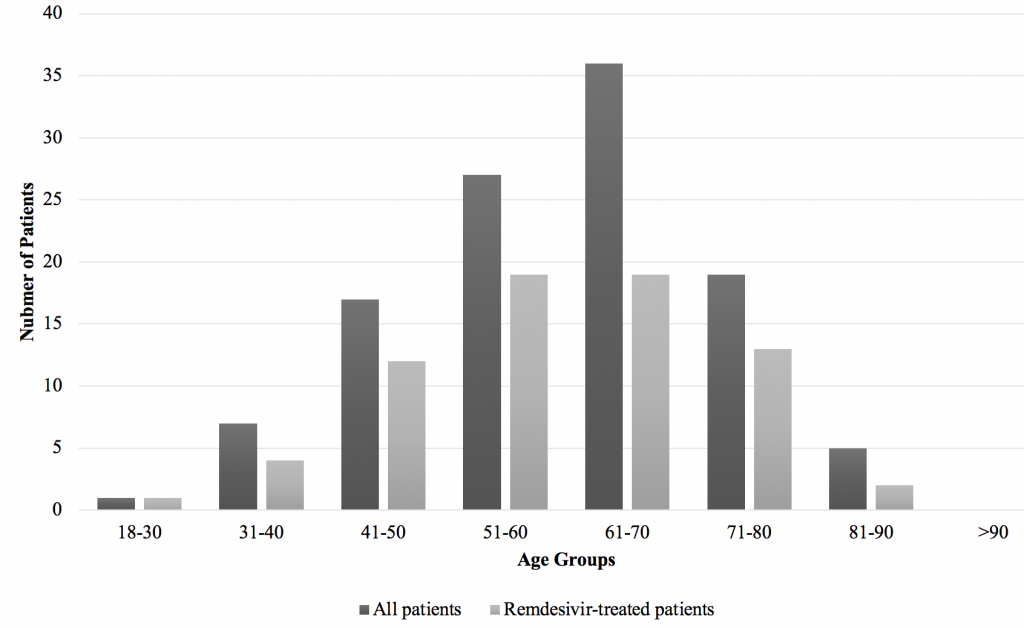
Table 1: Demographics of Covid-19 Patients Treated with Dexamesthasone only Versus Dexamethasone And Remdesivir
Total | Dexamethasone | Dexamethasone plus remdesivir | P value | |
N | 112 | 42 | 70 |
|
Age | 60±13 | 62±13 | 60±13 |
|
Percent mortality | 27% | 19% | 31% | 0.1655 |
Days to treatment | 6±4.4 | 7.1±5.7 | 5.3±3.3 |
|
Oxygen delivery at onset of treatment |
| |||
Room air | 10% | 14% | 7% | 0.2261 |
Low flow cannula | 54% | 67% | 46% | 0.0317 |
HiFlo cannula | 30% | 14% | 40% | 0.0039 |
Intubation | 6% | 5% | 7% | 0.6734 |
Comorbidities |
| |||
Diabetes | 62% | 34% | 64% | 0.0022 |
Hypertension | 52% | 34% | 49% | 0.1226 |
Obesity | 47% | 40% | 53% | 0.1845 |
COPD | 7% | 7% | 4% | 0.4877 |
Demographics of patients treated with either dexamethasone only (n=42) or dexamethasone and remdesivir (n=70). *Days to treatment is defined as time from symptom onset to the beginning of dexamethasone therapy.
Table 2: Demographics of Patients Categorized by Mortality Outcome and Pharmacotherapy
Death n=30 | Survival n=82 | |||
Dexamethasone | Dexamethasone plus remdesivir | Dexamethasone | Dexamethasone plus remdesivir | |
Age | 67±11 | 62±15 | 60±13 | 59±12 |
Days to death or recovery | 20.2±15.7 | 14.4±3.2 | 16.4±10.7 | 19.1±12.5 |
Days to treatment* | 7.1±4.6 | 4.6±3.5 | 7.1±5.9 | 5.6±3.2 |
Oxygen delivery at onset of treatment | ||||
Room air | 13% | 9% | 15% | 6% |
Low flow cannula | 50% | 32% | 71% | 52% |
HiFlo cannula | 25% | 41% | 12% | 40% |
Intubation | 13% | 18% | 3% | 2% |
Comorbidities | ||||
Diabetes | 63% | 59% | 56% | 67% |
Hypertension | 75% | 64% | 53% | 42% |
Obesity | 50% | 45% | 35% | 56% |
COPD | 38% | 0% | 6% | 6% |
Comparing the demographics of patients depending on their mortality outcome and pharmacotherapy for COVID-19. *Days to treatment is defined as time from symptom onset to the beginning of dexamethasone therapy.
Table 3: Patient Demographics by Mortality Outcome
Death | Survival | |
n | 30 | 82 |
Age | 64±13 | 60±13 |
Days to death or recovery | 19±14 | 18±12 |
Days to treatment* | 6±4 | 6±5 |
Oxygen delivery at onset of treatment | ||
Room air | 10% | 10% |
Low flow cannula | 37% | 60% |
HiFlo cannula | 37% | 28% |
Intubation | 17% | 2% |
Comorbidities | ||
Diabetes | 60% | 62% |
Hypertension | 67% | 46% |
Obesity | 47% | 48% |
COPD | 10% | 6% |
Comparison of demographics of patients who expired during their COVID-19 hospitalization (n=30) and those who survived (n= 82).* Days to treatment is defined as time from symptom onset to the beginning of dexamethasone therapy.
We would like the to thank the Institutional Review Board at Arrowhead Regional Medical Center for their assistance in safely and appropriately acquiring data for this analysis. We would also like to thank our colleagues at the California University of Science & Medicine (CUSM) who helped us in this endeavor, particularly our psychometrician, Jason Crowley, for his assistance in data analysis.
Christina is a third-year medical student at the California University of Science & Medicine. She comes from a background in biological anthropology and healthcare project management, and has since developed her interests in surgery and surgical education. In her free time she enjoys writing, baking, and hiking in the Southern California area.
For more information about this article, please contact Christina Chopra at 1013 Whistle Stop Drive, Colton, CA 92324, 940.783.7958, ChopraC@calmedu.org.
Liliana Filipowska is a medical student at the California University of Science & Medicine School of Medicine.
Dr. Sharon Y. Wang is an infectious disease specialist in Colton, California and is affiliated with Arrowhead Regional Medical Center. She received her medical degree from College of Osteopathic Medicine of the Pacific.
HPHR.org was designed by ComputerAlly.com.
Visit HPHR’s publisher, the Boston Congress of Public Health (BCPH).
Email communications@bcph.org for more information.

Click below to make a tax-deductible donation supporting the educational initiatives of the Boston Congress of Public Health, publisher of HPHR Journal.![]()