Goodman M. Potential economic burden contributing to treatment nonadherence in end-stage renal disease . HPHR. 2021;54. 10.54111/0001/BBB2
There are over 750,000 patients in the United States with end-stage renal disease (stage 5 chronic kidney disease), a fatal disease which requires extensive treatment to prolong survival. Currently, 7% of the annual Medicare budget is for the end-stage renal disease population, though they comprise only about 0.1% of the United States population. Although patients with end-stage renal disease are covered under Medicare, nearly half of patients with end-stage renal disease are nonadherent to treatment; this outcome may be related to high treatment costs for those who cannot afford either coinsurance or deductibles. Treatment nonadherence is important, as it may exacerbate symptoms and death as well as generate further expenses. However, there remains gaps in our knowledge of how healthcare expenditures and other forms of cost play a role in nonadherence to treatment within the end-stage renal disease population. It is therefore necessary that we consider the potential monetary reasons that cause end-stage renal disease treatment nonadherence. In this paper, we will discuss components of end-stage renal disease costs—including renal replacement therapy, healthcare utilization, and employment opportunities—and speculate on their effects toward treatment nonadherence. In order to improve treatment adherence, it may be necessary for Medicare and the private health insurance companies to cover more of the expenses of care than they currently do or for the market to establish more affordable options for patients. Healthcare officials will need to be proactive in both government and private sectors for necessary cost reductions.
The prevalence of chronic kidney disease (CKD) worldwide and in the United States is 13% and 15% respectively, and CKD carries a significant economic burden (United States Renal Data System, 2021). As the disease progresses to stage 5 CKD (end-stage renal disease [ESRD]), or kidney failure, patients not only require dialysis treatment but also disproportionately face severe and costly (see Table 1) complications, including anemia, stroke, heart failure, and infarction, which increase in incidence substantially (Abramson, Jurkovitz, Vaccarino, Weintraub, & Mcclellan, 2003). CKD is predominantly caused by diabetes mellitus and hypertension, though other causes are genetic, trauma-based, cancer-induced, pathologic, and more (Kazancioğlu, 2013). As the global median age increases, as do many potential etiologies of CKD, the prevalence of CKD is expected to rise along with its cost burden to societies (Birbov, et al., 2020). Currently, ESRD alone is believed to cost $1 trillion worldwide and $49.2 billion in the United States (Wouters, O’Donoghue, Ritchie, Kanavos, & Narva, 2015; United States Renal Data System, 2021). Additionally, an estimate suggests that CKD may change from the 12th leading cause of death worldwide to the fifth by 2040 (Foreman, et al., 2018). It is therefore necessary that we recognize factors we can act on to minimize the incidence of ESRD as well as mitigate its severity and cost.
Almost a quarter of patients with ESRD are now surviving a decade or more following diagnosis because of modern medical advances; however, such advances in diagnoses and treatments come with exorbitant costs, as will be discussed (de Souza Ferreira, et al., 2020). While ESRD allows a patient under 65 years to be eligible for Medicare, patients must still pay deductibles and about 20% of the Medicare-approved amount for any covered dialysis-related services, with variations if the patients are on Medicare Advantage. Of significant note is that approximately half of patients with ESRD are nonadherent to treatment (Kutner, 2001). With ESRD requiring a great degree of medical care and compliance, it is suspected that nonadherence to ESRD treatment poses a substantially high medical risk as well as a high economic cost. In this article, some important economic factors that may play a role in CKD treatment nonadherence will be discussed, including general costs, dialysis and dialysis centers, healthcare utilization, the Medicare benefit gap, and employment barriers. Additionally, potential solutions within both the public and private economic sectors will be highlighted.
Table 1. CKD healthcare expenditures by comorbidity
Comorbidity | Cost | Sources |
Anemia | $1,089 monthly | (Lefebvre, Duh, Buteau, Bookhart, & Mody, 2006) |
$20,529 annually | (Ershler, Chen, Reyes, & Dubois, 2005) | |
$28,757 annually | (Nissenson, Wade, Goodnough, Knight, & Dubois, 2005) | |
$140,925 lifetime | (Yarnoff, et al., 2016) | |
Heart Failure | $31,063 first 4 months after incident | (Betts, et al., 2021) |
$46,948 annually | (United States Renal Data System, 2021) | |
Diabetes Mellitus | $35,122 annually | (United States Renal Data System, 2021) |
Stroke | $21,087 first 4 months after incident | (Betts, et al., 2021) |
Myocardial Infarction | $21,016 first 4 months after incident | (Betts, et al., 2021) |
CKD is made up of five stages, including ESRD (stage 5), and has an increasingly substantial burden on the patient and on healthcare systems. The stages of CKD are based on the kidneys’ glomerular filtration rate (GFR), with lower GFRs meaning advanced stages of CKD and worsening kidney damage. Note that only ESRD would make someone under 65 years eligible for Medicare and not any other stage of CKD. In the United States, the mean annual total healthcare costs increase to $16,692 after stage 2, $31,596 for stage 3b, and $53,186 for stages 4 and 5 before becoming $100,594 without dialysis during ESRD (Golestaneh, et al., 2017). These costs do not take into account non-healthcare costs and productivity losses, which is not well understood in CKD. Total medical costs for CKD in 2013 were more than $81 billion in the United States, higher than age-matched patients without CKD (Saran, et al., 2016). Currently, 7% of the annual Medicare budget is for ESRD alone, though only about 0.1% of the population is a patient with ESRD (United States Renal Data System, 2021). A troubling statistic is that total Medicare ESRD fee-for-service spending for beneficiaries increased by $9.2 billion, between 2009 and 2019, and outpatient costs increased 50% from 2009 to 2019 (United States Renal Data System, 2021). In the 21st century, there has been moderate growth in ESRD spending, which imposes a large societal burden and could contribute to nonadherence to treatment. Not only do more complications arise from CKD that require more necessary treatment than otherwise but also patients with ESRD must choose between different costly kidney replacement therapies (RRT).
The two types of RRT are peritoneal dialysis (PD) and hemodialysis (HD). HD is a blood and dialysis solution circuit apparatus that filters the blood of patients, typically through the arm, for a few hours about thrice weekly. PD infuses a solution into the peritoneal cavity and collects waste from the blood by using the peritoneal membrane as an exchanger on an ongoing but daily regimen. Each form of RRT has varying costs and burdens to the patient. For example, PD is 22.7% cheaper than HD (or $75,140 compared to $88,750), and lifetime healthcare costs $204,442 for PD compared to $237,795 for HD (Gansevoort, et al., 2013; United States Renal Data System, 2021). Between 2015 and 2016, HD care increased by $2 billion to $26.8 billion, and HD spending per person per year increased by $2,000 to $88,750 (United States Renal Data System, 2018). However, while HD total spending increased by 5.7%, PD spending per person per year increased by 1.4% during the same period of time. This is because HD has more outpatient care, which makes up 66% of its total costs, whereas 51% of PD costs are from dialysate fluids (Eriksson, Neovius, Jacobson, Elinder, & Hylander, 2016). Additionally, HD has high fixed costs from the HD machines and staff, with HD machines costing about $24,000 on average with about a decade life cycle, as well as variable factors including the dialyzers, maintenance, and transportation. PD costs are largely due to variable costs such as the dialysate (Just, et al., 2008). However, PD is generally reserved for those patients who feel comfortable or are independent enough to manage their dialysis care at home and could be trained, as there is a risk of contamination and severe infection. The cost of training could be over $7,000, and training is limited by region (Eggers, 2011). If patients with ESRD were to receive home HD, it would be about 55% cheaper than in-center hemodialysis, though home HD has the same complications as PD, with the additional home dialysis equipment and storage costs and training of over $16,000.
These costs can be tremendous for the ESRD population, for who only 23% to 24% are employed in the United States, and only about 40% are informed of therapeutic alternatives to their current treatment (Van Biesen, van der Veer, Murphey, Loblova, & Davies, 2014; Hallab & Wish, 2018). It may mean many of ESRD costs are from poor patient-informed consent. The lack of true informed consent that patients with ESRD receive may be due to the way dialysis centers discuss PD, as few nephrologists are trained in PD and PD management (Kaplan, 2017). It appears that when patients with ESRD are informed of their choices, more than 50% choose home-based treatments; currently, only about 10% of patients are dialyzed at home (Oliver, et al., 2007; United States Renal Data System, 2021). Of note is that a Taiwanese cross-sectional survey found HD and PD to have similar quality-adjusted life expectancy (Chang, et al., 2016). Another study stated that in-center dialysis provides the highest societal cost and lowest quality of life, whereas kidney transplantation provides the lowest societal cost and highest quality of life (Vanholder, et al., 2017). Additionally, the ESRD Treatment Choices (ETC) Model, which encourages home dialysis and kidney transplantation, was found to have an incremental cost effectiveness ratio of $67,528 per quality-adjusted life year to the United States healthcare system when compared to in-center dialysis (Cha, Zimmerman, & Hansen, 2020). Because of the short-term costs, stress, side effects, and potential lack of informed consent, rates of nonadherence to dialysis have been reported as high as 98% in one study (Childers, Dwosky, Kominski, & Maggard-Gibbons, 2019; Griva, et al., 2014). This rate is both alarming and very concerning as dialysis nonadherence can be deadly. Future studies and immediate action should take place to understand and mitigate the aforementioned factors that could encourage dialysis nonadherence.
It should be noted that in the United States, over two-thirds of patients with ESRD undergo dialysis in for-profit clinics run by two corporations (United States Renal Data System, 2021). For example, DaVita is one of the two largest outpatient for-profit dialysis operations in the United States and makes the majority of its profits through Medicare and Medicaid. However, commercial insurance generated 33% of their total revenue between 2010 and 2017 even though only about 10.5% of all their patients have commercial insurance (Erickson, et al., 2018). It is likely that the consolidation and merging of the dialysis market has adversely impacted patient care; one study found that if the Hirschman-Herfindahl Index, a measurement of market concentration, for dialysis clinics were to be reduced by 20% that over 8,000 fewer hospitalizations of patients with ESRD would occur in 1 year (Desai, et al., 2009). Profit status is complicated by healthcare coverage and adverse selection when a for-profit relies on patients to maintain their business (Gander, et al., 2019). For-profits are also found to have poorer transplantation rates and higher mortality (Chironda & Bhengu, 2016). The concern is that for-profit dialysis centers may be reluctant to refer a patient for transplantation, as doing so may cause a loss of revenue. This quandary not only increases the rate of mortality and decreases the number of patients with CKD who can be most effectively treated, but it also creates a less cost-effective healthcare system in our society. The effects of for-profit dialysis and nonadherence have not been studied yet and remain a gap in our knowledge.
In regard to complications, patients with CKD have a higher frequency than healthy patients of inpatient and outpatient visits. One study found that compared to normal patients, patients with CKD are almost twice as likely to be hospitalized for hip fracture and patients with ESRD are almost four times as likely, with median hospital costs increased by close to $1,000 and $5,000 for patients with CKD and ESRD respectively (Kim, Long, Montez-Rath, Leonard, & Chertow, 2016). However, most patients with CKD are admitted for hospitalization due to cardiovascular-related events because of their risk factors, including either hypotension or hypertension, obesity or emaciation, and infections (Imtiaz, et al., 2019). Note that expenditures due to emaciation and infections in CKD have not been studied. With patients with ESRD being hospitalized an average of twice a year, and 30% of patients with ESRD having an emergency hospitalization event after discharge, there exists a large financial burden in this patient population (Imtiaz, et al., 2019). The costs of treating a patient with CKD or ESRD for another disease process is more expensive due to additional medical and pharmaceutical considerations and a longer length of stay in the hospital; for example, treating patients with CKD for bone metastases is nearly $60,000 more expensive than patients without CKD (Puenpatom, Hull, McPheeters, & Schwebke, 2017). It has been reported that the stress of complications, declining health, and financial burden in ESRD may contribute to nonadherence (Kustimah, Siswadi, Djunaidi, & Iskandarsyah, 2019). Therefore, because a study has not yet explored the relationship of complications to nonadherence, it remains an important knowledge gap in the CKD and ESRD population.
To prevent, slow, and manage the progression of CKD to ESRD, medication is typically necessary. The average pill burden of patients undergoing dialysis is about 19 pills per day, offered by five different providers and consisting of about ten prescriptions—four pills per day is the national average for normal, healthy patients (Dodd, Palagyi, Guild, Jha, & Jan, 2018). Many of the drugs are antihypertensives, renin-angiotensin blockers, diuretics, beta-blockers, calcium channel blockers, alpha-blockers, erythropoiesis-stimulating agents, statins, supplements, and more (Prasad, et al., 2021). It is estimated that the average patient in the United States with CKD pays an annual out-of-pocket sum of $440, $381, or $569 for prescriptions depending if they have ESRD, utilize hemodialysis, or utilize peritoneal dialysis (United States Renal Data System, 2020). This adds to a national total of $3.5 billion. The high cost often leaves patients with CKD nonadherent, especially those of low income or who are uninsured, which may exacerbate symptoms; in fact, upwards to 80% of patients with CKD are nonadherent to their medications (Childers, Dwosky, Kominski, & Maggard-Gibbons, 2019). Additionally, what is concerning for patients with lower stages of CKD is that early access to medications can delay stage advancement and later higher-cost treatments like dialysis, but not being able to afford the out-of-pocket expenses can make this task difficult.
As it currently stands, the only potential cure for ESRD is a kidney transplant. In the United States, 71% of pediatric patients with ESRD receive a kidney transplant; however, that number falls to about 32.9% for patients aged 45 to 64 years, which is the majority of patients with ESRD (United States Renal Data System, 2010). Unfortunately, with how limited available kidneys are, 78.5% of patients with ESRD in a self-administered survey were willing to pay for a kidney from living donors, especially those patients with ESRD who were male, of poor health, and had household incomes of $50,000 or more. (Herold, 2010) While in the United States a kidney transplant may cost an average of about $81,000, it is less than 50% the total annual cost dialysis patients may incur (Knight, et al., 2015). Additionally, a kidney transplant adds 5.5 quality-adjusted life-years to the average patient, compared to 4 quality-adjusted life-years for a patient on dialysis, with each quality-adjusted life-year being $100,000 more cost-effective (Axelrod, et al., 2018). This becomes important in regards to nonadherence prior to transplantation, as patients with ESRD who are nonadherent have poorer outcomes after kidney transplantation (Cleemput, Kesteloot, Vanrenterghem, & De Geest, 2004). Nevertheless, a moral hazard exists in that the United States healthcare system lacks enough functional kidneys or the financial incentive to promote kidney transplantation (Johnson & Meyers, 2018). This is unfortunate for patients with ESRD, as their chances of minimizing costs from the disease decreases as they age, while many comorbidities, and hence subsequent healthcare costs, become more likely with age.
Treatment adherence due to costs may play a role in the diminished quality of life and severity of CKD as well. Medicare patients are reimbursed 75% of outpatient costs until they reach a “benefit gap,” which afterwards they pay 100%. A study found that patients with CKD become 20% less adherent to their treatment plans as they reach this benefit gap; they are also twice as likely to be nonadherent to their medications for other comorbid diseases (Park, et al., 2014). It may be interpreted that patients with CKD either wish to delay reaching the point of fully paying for all their medical costs or forfeit treatment as they near the benefit gap. However, with either scenario, delaying treatment can mean worsening health. In another study, more than 10% of participants in a survey were not able to take their medications as directed due to costs, with 35% of participants believing costs were “always a concern” (Kim, Long, Montez-Rath, Leonard, & Chertow, 2016). As Medicare Part D spending has risen 50% in patients with CKD and 80% in patients with ESRD between 2009 and 2018—due not only to the increased prevalence of the disease but also because of higher prescription prices—nonadherence may become more prevalent (United States Renal Data System, 2021). This is important, as medication nonadherence in the United States costs the healthcare system between $100 and $289 billion annually; but, it is unknown what percent of that is from the CKD and ESRD population (Viswanathan, et al., 2012).
With patients with CKD having a reduced ability to work due to their illness and treatments, employment can be an important factor in a patient’s quality of life and for affording healthcare expenditures. Not only does employment allow for economic productivity, but it also would lessen disability cost burden. In the United States, it is estimated that about a third of all dialysis patients are employed; the unemployed have four times the rate of mortality (Kutner, Zhang, Huang, & Johansen, 2010; Kim, et al., 2015; Hallab & Wish, 2018). Interestingly, employment declines in prodromal patients with HD 6 months prior to initiation, likely due to exacerbating symptoms and medical expenditures that precede ESRD while patients are in middle to late stages of CKD (Nie, et al., 2019). In regard to employment, the RRT modality and its circumstances are related. For example, it was found that employment is 25% more likely to be retained if the dialysis patients start RRT 6 months prior to job initiation and if dialysis is offered after 5:00 pm. However, a 2005 study found only about 18% of United States dialysis centers offered dialysis after 5:00 pm (2005 Summary Report of the End Stage Renal Disease (ESRD) Networks’ Annual Reports, 2005). Employment retainment is a little more likely if patients decide on home HD or PD care. However, dialysis at a for-profit had 11% odds of unemployment before the start of hemodialysis (Nie, et al., 2019). What is concerning is how dialysis treatment, which often coincides with nephrology consultation, can be so interrelated with prior employment; it is logical that those who are or wish to be employed desire a work-friendly treatment option. The only options are that patients either select an RRT that does not interfere with their employment, lose their employment and have difficulty paying for treatment, or forgo treatment and perish, all of which are difficult choices. Note that patients with ESRD survive about 7 days on average following discontinuation of dialysis (O’Connor, Dougherty, Harris, & Casarett, 2013).
Figure 1. Components of CKD expenditures
Figure 2. CKD cost reduction strategies by sector
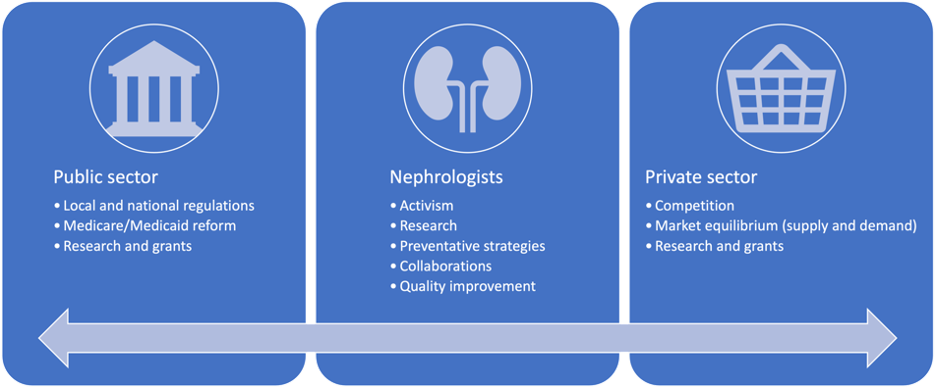
Overall, CKD places a heavy burden on patients’ quality of life and is a very costly disease for the individual and for the United States healthcare system. We have identified aspects of CKD’s various stages, particularly ESRD, and its comorbidities, treatments, and effects on employment as areas of high expenditures that establish the disease as being economically devastating. Within each of the topics that have been highlighted are avenues that may contribute to nonadherence to treatment. However, because we have not yet found the effects and economic costs of CKD and ESRD treatment nonadherence, there remains a significant knowledge gap in nephrology care that requires further exploration. Additionally, there are strategies, broad and specific, that various sectors of healthcare can take to reduce CKD costs (Figure 2). If studies are to find that treatment costs are having a significant impact on CKD and ESRD treatment nonadherence, it may be imperative that the federal government reduces the coinsurance required for ESRD care under Medicare, which the Affordable Care Act did beginning in 2010. Similar points can be made to private health insurance companies that cover patients with CKD but not ESRD. However, it is also necessary to further understand and then mitigate the rising costs of CKD and ESRD treatment so as to reduce the rates of treatment nonadherence. This is important primarily to those with CKD but not ESRD, as they may not be covered by Medicare and are therefore more susceptible to healthcare market prices. Achieving progress in these matters will necessitate nephrologists and other healthcare professionals to collaborate with government and private sectors. Otherwise, primary, secondary, and tertiary preventative strategies need to be further encouraged to reduce the incidence of CKD and hence its substantial costs.
2005 Summary Report of the End Stage Renal Disease (ESRD) Networks’ Annual Reports. (2005). Retrieved 7 2022, from Centers for Medicare & Medicaid Services (CMS): https://www.cms.gov/Medicare/End-Stage-Renal-Disease/ESRDNetworkOrganizations/downloads/NetworkAnnualReport2005.pdf
Abramson, J., Jurkovitz, C., Vaccarino, V., Weintraub, W., & Mcclellan, W. (2003). Chronic kidney disease, anemia, and incident stroke in a middle-aged, community-based population: The ARIC Study. Kidney Int., 64(2), 610-615. doi:10.1046/j.1523-1755.2003.00109.x.
Axelrod, D. A., Schnitzler, M. A., Xiao, H., Irish, W., Tuttle-Newhall, E., Chang, S. H., & al., e. (2018). An economic assessment of contemporary kidney transplant practice. Am J Transplant, 18(5), 1168-1176. doi:10.1111/ajt.14702.
Betts, K. A., Song, J., Faust, E., Yang, K., Du, Y., Kong, S. X., & Singh, R. (2021). Medical costs for managing chronic kidney disease and related complications in patients with chronic kidney disease and type 2 diabetes. Am J Manag Care, 27(20), S369-S374. doi:10.37765/ajmc.2021.88807.
Birbov, B., Purcell, C. A., Levey, A. S., Smith, M., Abdoli, A., & Abebe, M. E. (2020). Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet, 395(10225), 709-733. doi:10.1016/S0140-6736(20)30045-3.
Cha, A. S., Zimmerman, M., & Hansen, R. (2020). Puk4 Cost Utility Analysis Of End-Stage Renal Disease Treatment Choices (Etc) Model For Chronic Maintenance Dialysis In The United States. Value Health, 23. doi:10.1016/j.jval.2020.04.1463.
Chang, Y. T., Hwang, J. S., Hung, S. Y., Tsai, M. S., Wu, J. L., Sung, J. M., & Wang, J. D. (2016). Cost-effectiveness of hemodialysis and peritoneal dialysis: A national cohort study with 14 years follow-up and matched for comorbidities and propensity score. Sci Rep, 6, 30266. doi:10.1038/srep30266.
Childers, C. P., Dwosky, J. Q., Kominski, G., & Maggard-Gibbons, M. (2019). A Comparison of Payments to a For-profit Dialysis Firm From Government and Commercial Insurers. . JAMA Intern Med, 179(8), 1136-1138. doi:10.1001/jamainternmed.2019.0431.
Chironda, G., & Bhengu, B. (2016). Contributing Factors to Non-Adherence among Chronic Kidney Disease (CKD) Patients: A Systematic Review of Literature. Med Clin Rev, 2, 29. doi: 10.21767/2471-299X.1000038.
Cleemput, I., Kesteloot, K., Vanrenterghem, Y., & De Geest, S. (2004). The economic implications of non-adherence after renal transplantation. Pharmacoeconomics, 22(18), 1217-34. doi:10.2165/00019053-200422180-00006.
de Souza Ferreira, E., Moreira, T. R., da Silva, R. G., da Silva, L. S., de Oliveira Cavalier, S. B., Silva, B. O., . . . Cotta, R. M. (2020). Survival and analysis of predictors of mortality in patients undergoing replacement renal therapy: a 20-year cohort. BMC Nephrol, 21(1), 502. doi:10.1186/s12882-020-02135-7.
Desai, A. A., Bolus, R., Nissenson, A., Chertow, G. M., Bolus, S., Solomon, M. D., & Khawar, O. S. (2009). Is there “cherry picking” in the ESRD Program? Perceptions from a Dialysis Provider Survey. Clin J Am Soc Nephrol, 4(4), 772-777. doi:10.2215/CJN.05661108.
Dodd, R., Palagyi, A., Guild, L., Jha, V., & Jan, S. (2018). The impact of out-of-pocket costs on treatment commencement and adherence in chronic kidney disease: a systematic review. . Health Policy Plan, 33(9), 1047-1054. doi:10.1093/heapol/czy081.
Eggers, P. W. (2011). Has the incidence of end-stage renal disease in the USA and other countries stabilized? Curr Opin Nephrol Hypertens, 20(3), 241-245. doi:10.1097/MNH.0b013e3283454319.
Erickson, K. F., Zheng, Y., Ho, V., Winkelmayer, W. C., Bhattacharya, J., & Chertow, G. M. (2018). Market Competition and Health Outcomes in Hemodialysis. Health Serv Res, 53(5), 3608-3703. doi:10.1111/1475-6773.12835.
Eriksson, J. K., Neovius, M., Jacobson, S. H., Elinder, C. G., & Hylander, B. (2016). Healthcare costs in chronic kidney disease and renal replacement therapy: a population-based cohort study in Sweden. BMJ Open, 6(10), e012062. doi:10.1136/bmjopen-2016-012062.
Ershler, W. B., Chen, K., Reyes, E. B., & Dubois, R. (2005). Economic burden of patients with anemia in selected diseases. Value Health, 8(6), 629-638. doi:10.1111/j.1524-4733.2005.00058.x.
Foreman, K., Marquez, N., Dolgert, A., Fukutaki, K., Fullman, N., & McGaughey, M. e. (2018). Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. . Lancet, 392(10159), 2052-2090. doi:10.1016/S0140-6736(18)31694-5.
Gander, J. C., Zhang, X., Ross, K., Wilk, A. S., McPherson, L., Browne, T., & Pastan, S. O. (2019). Association Between Dialysis Facility Ownership and Access to Kidney Transplantation. JAMA, 322(10), 957-973. doi:10.1001/jama.2019.12803.
Gansevoort, R. T., Correa-Rotter, R., Hemmelgarn, B. R., Jafar, T. H., Heerspink, H. J., & Mann, J. F. (2013). Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. . Lancet, 382(9889), 339-352. doi:10.1016/S0140-6736(13)60595-4.
Golestaneh, L., Alvarez, P. J., Reaven, N. L., Funk, S. E., McGaughey, K. J., & Romero, A. e. (2017). All-cause costs increase exponentially with increased chronic kidney disease stage. Am J Manag Care, 23(10), 163-172.
Hallab, A., & Wish, J. B. (2018). Employment among Patients on Dialysis. Clin J Am Soc Nephrol, 13(2), 203-204. doi: 10.2215/CJN.13491217.
Herold, D. K. (2010). Patient willingness to pay for a kidney for transplantation. Am J Transplant, 10(6), 1394-1400. doi:10.1111/j.1600-6143.2010.03113.x.
Imtiaz, S., Qureshi, R., Hamid, A., A, H., Salman, B., & Drohlia, M. F. (2019). Causes of hospital admission of chronic kidney disease patient in a tertiary kidney care hospital. J Clini Nephrol, 3, 100-106. doi:10.29328/journal.jcn.1001033.
Johnson, D. S., & Meyers, K. B. (2018). Delaying and Averting Dialysis Treatment: Patient Protection or Moral Hazard? Am J Kidney Dis, 72(2), 251-254. doi:10.1053/j.ajkd.2018.01.042.
Just, P. N., de Charro, F. T., Tschosik, E. A., Noe, L. L., Bhattacharyya, S. K., & Riella, M. C. (2008). Reimbursement and economic factors influencing dialysis modality choice around the world. Nephrol Dial Transplant, 23(7), 2365-2373. doi:10.1093/ndt/gfm939.
Kaplan, A. A. (2017). Peritoneal Dialysis or Hemodialysis: Present and Future Trends in the United States. Contrib Nephrol, 189, 61-64. doi:10.1159/000450672.
Kazancioğlu, R. (2013). Risk factors for chronic kidney disease: an update. Kidney Int Suppl, 3(4), 368-371. doi:10.1038/kisup.2013.79.
Kim, J. M., Son, N. H., Park, E. C., Nam, C. M., Kim, T. H., & Cho, W. H. (2015). The relationship between changes in employment status and mortality risk based on the Korea Labor and Income Panel Study (2003-2008). Asia Pac J Public Health, 27(2), 993-1001. doi:10.1177/1010539513486923.
Kim, S. M., Long, J., Montez-Rath, M., Leonard, M., & Chertow, G. M. (2016). Hip Fracture in Patients With Non-Dialysis-Requiring Chronic Kidney Disease. . J Bone Miner Res, 31(10), 1803-1809. doi:10.1002/jbmr.2862.
Knight, T., Schaefer, C., Krasa, H., Oberdhan, D., Chapman, A., & Perrone, R. D. (2015). Medical resource utilization and costs associated with autosomal dominant polycystic kidney disease in the USA: a retrospective matched cohort analysis of private insurer data. Clinicoecon Outcomes Res, 7, 123-132. doi:10.2147/CEOR.S75523.
Kustimah, K., Siswadi, A. G., Djunaidi, A., & Iskandarsyah, A. (2019). Factors Affecting Non-Adherence to Treatment in End Stage Renal Disease (ESRD) Patients Undergoing Hemodialysis in Indonesia. Open Psychol J, 12, 141-6. doi:10.2174/1874350101912010141.
Kutner, N. (2001). Improving Compliance in Dialysis Patients: Does Anything Work? Semin Dial, 14(5), 324-327. doi:10.1046/j.1525-139x.2001.00080.x.
Kutner, N. G., Zhang, R., Huang, Y., & Johansen, K. L. (2010). Depressed mood, usual activity level, and continued employment after starting dialysis. J Am Soc Nephrol, 5(11), 2040-5. doi:10.2215/CJN.03980510.
Lefebvre, P., Duh, M. S., Buteau, S., Bookhart, B., & Mody, S. H. (2006). Medical costs of untreated anemia in elderly patients with predialysis chronic kidney disease. J Am Soc Nephrol, 17(12), 3497-3502. doi:10.1681/ASN.2006030289.
Nie, Y., Witten, B., Schatell, D., Assari, S., Ding, X., Saran, R., & Bragg-Gresham, J. L. (2019). Changes in employment status prior to initiation of maintenance hemodialysis in the USA from 2006 to 2015. Clin Kidney J, 13(3), 434-441. doi:10.1093/ckj/sfz077.
Nissenson, A. R., Wade, S., Goodnough, T., Knight, K., & Dubois, R. W. (2005). Economic burden of anemia in an insured population. J Manag Care Pharm, 11(7), 565-574. doi:10.18553/jmcp.2005.11.7.565.
O’Connor, N. R., Dougherty, M., Harris, P. S., & Casarett, D. J. (2013). Survival after Dialysis Discontinuation and Hospice Enrollment for ESRD. Clin J Am Soc Nephrol, 8(12), 2117-22. doi: 10.2215/CJN.04110413.
Oliver, M. J., Quinn, R. R., Richardson, E. P., Kiss, A. J., Lamping, D. L., & Manns, B. J. (2007). Home care assistance and the utilization of peritoneal dialysis. Kidney Int, 71(7), 673-8. doi:10.1038/sj.ki.5002107.
Park, H., Rascati, K. L., Lawson, K. A., Barner, J. C., Richards, K. M., & Malone, D. C. (2014). Adherence and persistence to prescribed medication therapy among Medicare part D beneficiaries on dialysis: comparisons of benefit type and benefit phase. J Manag Care Spec Pharm, 20(8), 862-76. doi:10.18553/jmcp.2014.20.8.862.
Prasad, N., Yadav, A. K., Kundu, M., Sethi, J., Jaryal, A., & Sircar, D. (2021). Prescription Practices in Patients With Mild to Moderate CKD in India. Kidney Int Rep, 6(9), 2455-2462. doi:10.1016/j.ekir.2021.06.011.
Puenpatom, A., Hull, M., McPheeters, J., & Schwebke, K. (2017). isease Burden, Early Discontinuation, and Healthcare Costs in Hepatitis C Patients with and without Chronic Kidney Disease Treated with Interferon-Free Direct-Acting Antiviral Regimens. . Clin Drug Investig, 37(7), 687-697. doi:10.1007/s40261-017-0526-z.
Saran, R., Li, Y., Robinson, B., Abbott, K. C., Agodoa, L. Y., Ayanian, A., & Bragg-Gresham, J. (2016). US Renal Data System 2015 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis, 67(3 suppl 1), 1-434. doi:10.1053/j.ajkd.2015.12.014.
United States Renal Data System. (2010). 2010 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. Retrieved from https://render.usrds.org/atlas10.aspx
United States Renal Data System. (2018). 2018 USRDS ANNUAL DATA REPORT. Retrieved from https://www.usrds.org/media/1734/v2_c09_esrd_costs_18_usrds.pdf
United States Renal Data System. (2020). 2020 Annual Data Report. Retrieved from https://adr.usrds.org/2020
United States Renal Data System. (2021). 2021 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. Retrieved from https://adr.usrds.org/2020/end-stage-renal-disease/10-prescription-drug-coverage-in-patientswith-esrd
Van Biesen, W., van der Veer, S., Murphey, M., Loblova, O., & Davies, S. (2014). Patients’ Perceptions of Information and Education for Renal Replacement Therapy: An Independent Survey by the European Kidney Patients’ Federation on Information and Support on Renal Replacement Therapy. PLoS ONE, 9(7), e103914. doi:10.1371/journal.pone.0103914.
Vanholder, R., Annemans, L., Brown, E., Gansevoort, R., Gout-Zwart, J. J., Lameire, N., & Morton, R. L. (2017). Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat Rev Nephrol, 13(7), 393-409. doi:10.1038/nrneph.2017.63.
Viswanathan, M., Golin, C. E., Jones, C. D., Ashok, M., Blalock, S. J., & Wines, R. C. (2012). Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med, 157(11), 785-95. doi:10.7326/0003-4819-157-11-201212040-00538.
Wouters, O., O’Donoghue, D., Ritchie, J., Kanavos, P., & Narva, A. (2015). Early chronic kidney disease: diagnosis, management and models of care. Nat Rev Nephrol, 11(8), 491-502. doi:10.1038/nrneph.2015.85.
Yarnoff, B. O., Hoerger, T. J., Simpson, S. A., Pavkov, M. E., Burrows, N. R., Shrestha, S. S., . . . Zhuo, X. (2016). The Cost-Effectiveness of Anemia Treatment for Persons with Chronic Kidney Disease. PLoS One, 11(7), e0157323. doi:10.1371/journal.pone.0157323.
Max Goodman is a medical student pursuing a dual degree MD/MS at the Medical College of Wisconsin. His primary research interest is understanding the effects of hemodialysis on brain structure integrity in patients with end-stage renal disease under the mentorship of Dr. Dawn Wolfgram. He is also investigating blood-based biomarkers in Alzheimer’s disease as well as ways of improving global health connections amongst medical students.
HPHR.org was designed by ComputerAlly.com.
Visit HPHR’s publisher, the Boston Congress of Public Health (BCPH).
Email communications@bcph.org for more information.

Click below to make a tax-deductible donation supporting the educational initiatives of the Boston Congress of Public Health, publisher of HPHR Journal.![]()