Walker M. Bower E. Iyiegbuniwe E. Development of quality measures for enhanced preventive screening of women for cervical cancer. HPHR. 2021;52.10.54111/0001/ZZ1
In this study, we reviewed screening methods for the development of quality measures for enhanced preventive screening of women for cervical cancer. The World Health Organization has identified cervical cancer as a global health crisis and the fourth most common cancer in women. Recent developments in cervical cancer research have focused on the lack of new and accessible methods of detection and the desire to provide alternate screening methods since the benefits of the early preventive screening would outweigh potential harms. Analysis of newer methodologies and developments in preventive screening for cervical cancer is of significant public health importance in reducing the current prevalence and mortality rates worldwide. Self-sampling tests for cervical cancer are a viable option for affordable and accessible testing. We searched different electronic databases and the articles that met all the inclusion and exclusion criteria were included in this review. Articles were grouped into three themes: global prevalence of cervical cancer, current screening methods, and development of quality measures for enhanced screening. The results of this review showed that a wide gap still exists in the literature regarding the development of adequate, accessible, and preventive screening, diagnostic, and treatment methods for cervical cancer. More accurate and precise self-sampling and screening tests with higher acceptability and accessibility rates for HPV must be developed to detect cervical dysplasia and cervical cancer with the intention of widespread implementation. There is an urgent need to foster strong partnerships with all stakeholders, particularly decision makers to inform policy and establish newly improved, timely, and accurate screening results to reduce the disproportionate mortality rates due to cervical cancer among minority women across the United States.
Cervical cancer is a global health crisis and the fourth leading cause of cancer in women worldwide.¹ The mortality rate attributed to cervical cancer is 18 times higher in Low and Middle Income Countries (LMICs) than in wealthier countries.² Wealth is a strong determining factor when considering the benefits of cervical cancer screening, as evidenced by the disparity in mortality rates between areas of high and low socioeconomic status (SES). In an effort to decrease the number of deaths each year, new developments in cervical cancer screening are being tested and implemented across the globe.² Recent developments in cervical cancer screening focus primarily on developing new and accessible preventive screening measures.
Every year in the United States, approximately 60,000 mothers die and suffer from pregnancy-related diseases or medical conditions³ and 311,000 deaths were linked to cervical cancer worldwide.⁴ Furthermore, empirical evidence showed that higher maternal mortality and outcomes due to the risk factors were more prevalent among Blacks, American Indian/Alaska Native, and rural dwellers. The United States Preventive Services Task Force (USPSTF) has reported that racial and ethnic minority women have disproportionately higher burdens, incidence and mortality rates of cervical cancer.⁵ The overall cervical cancer mortality rate in the United States is 2.3 per 100,000 women, with the highest mortality rate of 3.7 per 100,000 in African American women.⁵
Worldwide, vaccination against Human Papillomavirus (HPV) is considered the primary prevention method against cervical cancer and 124 countries have implemented national immunization programs for HPV.⁶ The HPV vaccine protects vaccinated women against the two major oncogenic types of HPV known to cause cervical cancer.
The Coronavirus Disease 2019 (COVID-19) pandemic has had a significant impact on the number of women being screened for cervical cancer. The rates of cervical cancer screenings declined among minority, low-income women with delays in screening increasing existing health disparities in these populations.⁷ In the United States, the National Breast and Cervical Cancer Early Detection Program found that between January and June 2020, cervical cancer screening declined by 84%.⁷ In early April 2020, an 80% drop in weekly mammography and cytology tests were reported by insurance companies, indicating a lack of screening during COVID-19.⁷ Delayed screening during COVID-19 may contribute to later diagnosis and delayed treatment of more serious and advanced cervical cancer cases.⁷
In the United States, current guidelines and recommendations for prevention and early detection of cervical cancer are identified by the American Cancer Society⁸ and the USPSTF.⁵ The three methods currently available for cervical cancer screening tests include Cytology, primary HPV tests, and Cotests.⁹ Cytology test is the primary screening method, however, cytology alone offers slightly less sensitivity in detecting abnormal cells that cause cervical cancer.¹⁰ HPV testing offers greater sensitivity for the detection of the 12 genotypes of HPV.¹¹ Cotest screening consists of both cervical cytology and high-risk HPV (hrHPV) testing and can detect high-grade precancerous cervical lesions.⁸,⁹
Self-sampling is a screening tool that has been used for cytology or HPV testing, but has not been approved by the U.S. Food and Drug Administration (FDA).⁹ A major benefit of self-sampling is that hrHPV testing samples can be collected by the patient thus increasing screening rates among populations with limited accessibility to clinics or hospitals.⁵ It is anticipated that self-sampling will play a prominent role in cervical cancer screening as more research and supporting evidence are conducted.⁹
HPV self-sampling has the potential to increase uptake of cervical cancer screening.¹² HPV polymerase chain reaction (HPV-PCR) is a widely used screening method. Newer HPV-PCR technology can be performed rapidly, is more sensitive, and at lower cost, hence allows for higher-volume screening for cervical cancer.¹³ In addition, HPV genotyping has been shown to be a major predictor of progression and as being clinically important for cervical cancer management and risk estimation.¹¹
Recent advances in high resolution digital colposcopy (DC) have been used in HPV screening for the detection of cancerous cervical lesions and may offer enhanced imagining and magnification for rapid, portable, and cost-effective cervical cancer screening.¹³ However, screening and treatment of precancerous cells using colposcopy with biopsy links have been shown to be diagnostically poor and subjective. Dynamic spectral imaging cervical mapping has been used to standardize colposcopy and quantify cervical acetowhitening and is considered supportive of colposcopic assessment and biopsy placement.¹⁴
We searched electronic databases for the following keywords: quality measures, preventive screening, cervical cancer, Human Papillomavirus (HPV). The inclusion criteria were full text articles published in English language from 2016 to 2021 and adult women aged 18 – 65 years. Articles that did not meet the inclusion criteria were excluded. The selection process carefully followed the search guidelines outlined by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).¹⁵
Our initial search returned 1,042 articles meeting the inclusion and exclusion criteria. Sixty-one articles were reviewed following a title record screening. Of the 61 articles reviewed, 21 were included in the review. The 21 articles that met the inclusion and exclusion criteria are summarized in Figure 1 using the PRISMA chart.¹⁵
Figure 1. Flowchart of selection process and search results (adapted from PRISMA protocol)¹⁷
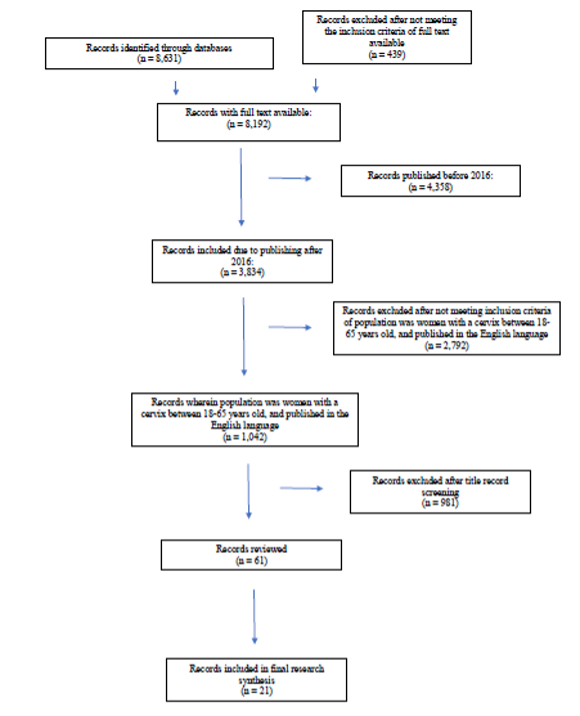
The articles in this review were categorized into three themes based on research questions, major findings, conclusions, and study limitations and are summarized in Table 1. The three themes were: global prevalence of cervical cancer, current screening methods, developments of quality measures for enhanced screening.
Table 1. An Overview of Selected Articles (Summary Results, Conclusion, and Themes).
Reviewed Article | Results and Conclusion | Theme |
Small, W., Bacon, M. A., Bajaj, A., Chuang, L. T., Fisher, B. J., Harkenrider, M. M., Jhingran, A., Kitchener, H. C., Mileshkin, L. R., Viswanathan, A. N., & Gaffney, D. K. (2017). Cervical cancer: A global health crisis. Cancer, 123(13), 2404–2412. https://doi.org/10.1002/cncr.30667 | Cervical cancer is the fourth most frequently occurring malignancy in women worldwide, with 530,000 new cases and 270,000 deaths reported annually. Tumors in the ectocervix were squamous cell carcinomas (~75% of invasive cervical carcinoma cases) whereas tumors from the endocervix were more likely to be adenocarcinomas. Vaccination was considered a primary prevention and screening, a secondary prevention. HPV testing was more sensitive than cytology, and the screening intervals for HPV-negative women can be safely extended. | Global Prevalence of Cervical Cancer |
U.S. Preventive Services Task Force. (2018). Evidence summary. Cervical Cancer: Screening. https://www.uspreventiveservicestaskforce.org/uspstf/document/evidence-summary/cervical-cancer-screening | Screening with cytology alone, hrHPV testing alone or cotesting can detect high-grade precancerous cervical lesions and cervical cancer. False-negative results for invasive cervical cancer were uncommon and false-positive results were two times higher with cotesting. | Current Screening Methods |
Niu, L., Virani, S., Bilheem, S. & Sriplung, H. (2019, November The effect of Pap smear screening on cervical cancer stage among southern Thai women. Sci Rep (9)16921. https://doi.org/10.1038/s41598-019-52607-6 | Patients are more likely to be diagnosed at an early stage with more frequent pap smears. The odds of screened women with late-stage diagnoses was 0.43 times lower than non-screened women. The probability of being diagnosed at a late stage increases rapidly among females aged 40 to 55 years. The authors reported that stage of cancer at diagnosis was a critical determinant of cancer outcomes and is directly associated with survival rates in cancer patients. | Current Screening Methods |
Fontham, E. T. H., Wolf, A. M. D., Church, T. R., Etzioni, R., Flowers, C. R., Herzig, A., Guerra, C. E., Oeffinger, K. C., Shih, Y.-C. T., Walter, L. C., Kim, J. J., Andrews, K. S., DeSantis, C. E., Fedewa, S. A., Manassaram-Baptiste, D., Saslow, D., Wender, R. C., & Smith, R. A. (2020). Cervical cancer screening for individuals at average risk: 2020 Guideline update from the American Cancer Society. CA: A Cancer Journal for Clinicians, 70(5), 321-346. https://acsjournals.onlinelibrary.wiley.com/doi/full/10.3322/caac.21628. | Primary HPV testing was shown to improve early detection. The disease burden of cervical cancer among individuals aged <25 years was low. For the average at-risk individuals, screening for cervical cancer was initiated at 25 years of age rather than 21 years, and primary HPV testing was preferred.Cytology testing was shown to have inferior sensitivity and provided lesser assurance regarding future risks when compared with primary HPV testing. A combination of cytology and HPV testing (co-testing) offered very little incremental benefit in detection and was more risky. The authors noted that primary HPV testing should be designated as the preferred screening test. | Current Screening Methods |
Nishimura, H., Yeh, P. T., Oguntade, H., Kennedy, C. E., & Narasimhan, M. (2021). Hpv self-sampling for cervical cancer screening: A systematic review of values and preferences. BMJ Global Health, 6(5). https://doi.org/10.1136/bmjgh-2020-003743 | Women generally prefered self-sampling to and found HPV self-sampling highly acceptable than clinician sampling regardless of age, income or country of residence. Also, most women preferred home-based self-sampling to self-sampling at a clinic. Lack of self-confidence with collecting a reliable sample was the most commonly cited reason for preferring clinician-collected samples. The most frequently cited reasons included less pain or physical discomfort, ease of use, convenience, ability to perform the test in private, and less embarrassment or anxiety. | Developments of Quality Measures |
Norenhag, J., Du, J., Olovsson, M., Verstraelen, H., Engstrand, L., & Brusselaers, N. (2020) The vaginal microbiota, human papillomavirus and cervical dysplasia: a systematic review and network meta-analysis. BJOG, An International Journal of Obstetrics and Gynecology. 127(20), 171-180. https://obgyn.onlinelibrary.wiley.com/doi/abs/10.1111/1471-0528.15854 | Vaginal microbiota dominated by non-Lactobacilli species or Lactobacillus iners were associated with 3-5x times higher odds of any prevalent HPV and 2-3x times higher for hrHPV and dysplasia/cervical cancer compared with Lactobacillus crispatus. | Developments of Quality Measures |
The World Health Organization (2020) has identified cervical cancer as a global health problem with 85 percent of reported cases occurring in LMICs¹. Cervical cancer causes an estimated 530,000 new cases and 270,000 deaths annually.²,¹⁶ A study by Small et al.² noted that a global call to action against cervical cancer in low-income and middle-income countries was desperately needed. Countries that do not currently have nationwide organized screening methods expect that the diagnostic performance of screening and the implementation system of the screening will be considerably low, resulting in decreased coverage rates of cervical cancer screening.¹⁷
The highest cervical cancer mortality rate in the United States is among African Americans (3.7 per 100,000 women) compared to the national average (2.3 per 100,000 women).⁵ Women between the ages of 44-55 years experience the highest rates of late-stage cervical cancer diagnoses.⁴ Women of low SES also experience higher rates of cervical cancer.⁹
A recent study by Wentzensen¹⁸ showed that cervical cancer can be prevented or detected early through regular screenings and follow-up treatments. Successful screening and management of cervical cancer precursors have led to substantial reductions in cervical cancer incidence and mortality rates.⁹ These improvements have allowed for more accurate predictions of individuals diagnosed with cervical pre-cancer and cancer.¹⁸ The USPSTF⁵ demonstrated convincing evidence that screening with cytology alone, primary testing for hrHPV alone, or cotesting can detect high-grade precancerous cervical lesions and cervical cancer. Additionally, compared with primary HPV testing, cytology testing was shown to have inferior sensitivity and less assurance regarding future risk for cervical cancer. A combination of cytology and HPV testing (cotesting) offered very little accuracy⁹ and patients were more likely to be diagnosed at an early stage with more frequent cytology testing.⁴
Bedell et al.¹³ reported that HPV polymerase chain reaction (PCR) testing, digital colposcopy technology and visual inspection with acetic acid or visualization with Lugol’s iodine have been reported to be cost-effective and accurate screening methods in resource-poor settings. The authors noted that the ability to screen many more women using self-collected samples was a good justification for the small decrease in testing accuracy.¹³
A recent study by Nishimura et al.¹⁹ showed that self-sampling is a highly acceptable method for HPV testing, regardless of study location, sampling method, device, setting, or participant’s demographics. Their results showed that higher levels of acceptability were consistent among vulnerable and under-screened subpopulations.¹⁹ Additionally, HPV self-sampling had the potential to increase uptake of cervical cancer screening. However, follow-up clinical assessment or treatment was reported to be low and did not provide any advantage when compared to other methods of cervical cancer screening.²⁰
Norenhag et al.²¹ reported that vaginal microbiota dominated by Lactobacillus iners were associated with three to five times higher odds of detection of any prevalent HPV and two to three times higher detection for hrHPV and dysplasia/cervical cancer when compared with Lactobacillus crispatus. The authors noted that this would be useful for guiding treatment options or in serving as biomarkers for HPV-related diseases.²¹
The greatest effects on decreasing the prevalence and mortality rates of cervical cancer can be accomplished by providing accessible, affordable screenings to women who are under-screened and unscreened. All screening tests for cervical cancer must be closely followed by diagnostic testing and treatment. A number of studies have shown that HPV-DNA and hrHPV self-sampling are promising newly developed strategies for increasing the accessibility and accuracy of screening rates for women living in LMICs.¹²,¹⁹ Evaluation is required to determine whether self-sampling is an effective method of collection and detection as well as how they will be implemented.⁵ The implementation of the applicable strategies and accessible follow-up care must closely follow the testing of self-sampling for women in LMICs.
In the United States, the risk factors and higher mortality rates for cervical cancers were more prevalent among Blacks, American Indian/Alaska Native, and other minority populations.⁵ The risk factors that contribute to these inequities have been attributed to differential participation and follow up in screening programs, health seeking behaviors, and treatment access barriers.⁹ The cervical cancer data for women in minority populations closely correlates with the higher incidence and mortality rates among women with lower SES.⁹ Lack of insurance is a contributing factor to lower screening rates in low SES populations as it inhibits women from being screened and prevents further diagnostic testing and treatment for cervical cancer and cervical dysplasia.⁹
Recommendations for guidance on cervical cancer screening vary from one country to the next. In the United States, the USPSTF provides screening recommendations and The American Cancer Society⁸ provides recommended guidelines that all physicians must follow. Currently, the guidelines suggested by these organizations stipulate that women should begin screening at the ages of 21-25 years and cease screening at age 65. This is due in part to the fact that benefits of screening before and after the above age groups are not significant and do not outweigh any potential harms. Inadequate screening at a young age and not screening before the recommended age group can lead to late diagnoses and the development of cervical cancer at older ages.⁹
Lack of adherence to regular screening protocols for the recommended age groups can be addressed by more efficient follow-up and accessible screening methods for both older and younger women. In LMICs, failure to initiate screening and lack of screening as individuals approach the age where screening is no longer recommended, due to the benefits ceasing to outweigh the harms, can be prevented using strategies that assist in increasing screening rates. The recommended strategies include increasing access to screening, encouraging follow up diagnostic treatment, and promoting equal participation in screening.
Low cervical cancer screening rates are a strong predictor of high mortality rates in populations with little or no access to screening services.¹⁰ In addition, disparities in follow-up of cases and treatment protocols are strong contributing factors. Developing systems that are more proficient in the follow-up of abnormal results and treatment are needed.¹⁰ There is an emphasis on developing different, increasingly accessible screening methods in the cervical cancer field; however, there is a large gap in research regarding the development of accessible screening, diagnostic tests, and treatment. Follow-up is an essential part of reducing cervical cancer rates and must be addressed in future research.
Cervical cancer screening strategies have the potential to be reevaluated due to the decrease in hrHPV type prevalence following vaccination practices and the introduction of primary HPV testing. Considering that a large majority of cervical cancer screening practices are based on HPV screening, the wide implementation of hrHPV vaccines requires that current screening practices must be changed or improved.19,21 The decrease in hrHPV type and potential decrease in cancer incidence may increase the number of false-positive results, which may balance the benefits and harms of current screening practices.⁵ In recent years, primary HPV testing as a screening tool for cervical cancer has been approved in the United States⁸ and cotesting has increased, but it is still too early to measure utilization rates.⁹ The need for new screening strategies for cervical cancer and cervical dysplasia unrelated to HPV has become increasingly relevant as HPV vaccines become more widely available. The gap in knowledge around factors contributing to cervical cancer other than HPV must be addressed in order to improve the effectiveness of screening practices.⁹
Self-sampling used for HPV screening has great potential to expand the scope of cervical cancer screening in LMICs and under screened minorities in the United States. Acceptability of HPV self-sampling must be examined throughout LMICs.²⁰ Contextual factors surrounding HPV and cervical cancer may contribute to the acceptability of self-sampling and will be essential to successful implementation. Self-sampling may be experienced differently by women in LMICs due to poor medical care and less access to screening, diagnostic care, treatment, and information. It is important to consider the behavioral and social determinants of health when attempting to implement self-sampling in LMICs. The development of educational and other supporting materials will be an essential part of the movement towards self-sampling in LMICs and areas with poor access to screening tests to ensure that these methods are acceptable and adequate for the target population. Further research should focus on assessing the population of affected women in LMICs and provide insight into how the existing gaps in screening tests between higher and lower socioeconomic populations can be bridged in the near future.
Strengths of this study are the comprehensive search for relevant data and the broad scope of the study objective. Databases searched had access to a wide variety of data and the search was not limited by lack of access to multiple sources of data. Using listed keywords and key phrases to search for articles returned many peer-reviewed articles. To narrow the search, researchers used a restrictive inclusion and exclusion criteria that may have excluded articles with relevant data. The age restriction listed may have excluded articles that highlighted the development of quality measures for enhanced cervical screening of women under the age of 18 and over the age of 65. This is considered a strength because there is little benefit to screening women under the age 18 and over the age of 65.⁵,⁸ The language restriction listed in the inclusion criteria may have excluded articles with valuable perspectives from different countries and their databases. The restriction on the study publication year may have inhibited the inclusion of relevant data collected before 2016. The narrow inclusion and exclusion criteria is viewed as a strength because it allowed for the collection of the most relevant data. The benefits of having a narrow search criterion for the purpose of this review outweighed the drawbacks.
A limitation of the review is that it cannot easily be replicated by other researchers due to the authors’ decision-making process in determining which articles included relevant data. The authors’ decision-making process required debate within the authors themselves and many articles were restricted due to the perception of their relevance. The authors’ perspectives on relevance may significantly differ from other researchers attempting to replicate this review.
Cervical cancer constitutes a public health crisis around the globe and the mortality rate due to cervical cancer is significantly higher in LMICs than in wealthier countries. In the United States, cervical cancer constitutes an ongoing and challenging public health crisis. To address the multifaceted nature of this challenge, several factors must be considered, including the wide disparities in mortality burden that affects predominantly low-income minority women. This study was conducted to review the literature and summarize data on the prevalence and mortality rates with a view to developing quality measures for enhanced preventive screening for cervical cancer.
Cervical cancer screening is preventative in detecting precancerous cells. Screening measures detecting precancerous cells and cervical cancer cells include cervical cytology alone, primary testing for high-risk HPV types alone, or co-testing. HPV was shown to be the most prevalent indicator in the diagnosis of cervical cancer. Self-sampling is a highly acceptable, low cost, and convenient method of screening for HPV especially among vulnerable and disproportionately under-screened subpopulations. Emerging developments in cervical cancer screening consist of new self-sampling tests for HPV and cytology, newer HPV polymerase chain reaction technology, high resolution digital colposcopy using artificial intelligence, and research on the community types for vaginal microbiota. Other new technology screening developments included visual inspection with acetic acid or visualization with Lugol’s iodine.
Analyzing vaginal microbiota and identifying the dominance of specific strains (e.g., Lactobacillus iners) can lead to detection of HPV, hrHPV, cervical dysplasia, and cervical cancer. These new developments in screening could be viable options for increasing early detection of cervical cancers. Due to the high mortality rate of cervical cancer across the globe, prevention is highly recommended. In the near future, clinicians should expect multiple developments in screening technology that will decrease the prevalence and mortality rates of cervical cancer.¹²
The authors would like to thank the following individuals for their contributions to this project: Elizabeth S. Cortez and Monica J. Lashley.
The author(s) have no relevant financial disclosures or conflicts of interest.
1. Global strategy to accelerate the elimination of cervical cancer as a public health problem. World Health Organization. November 17, 2020. Accessed October 10, 2021. https://www.who.int/publications/i/item/9789240014107.
2. Small W, Bacon M, Bajaj A, Chuang L, et al. Cervical cancer: A global health crisis. ACS Journals. 2017;123(13): 2404–2412. https://doi.org/10.1002/cncr.30667. Accessed October 2, 2021.
3. Crear-Perry J, Correa-de-Araujo R, Johnson T L, McLemore M R, Neilson E, Wallace M. Social and structural determinants of health inequities in maternal health. Journal of Women’s Health. 2021;30(3):230-235. https://doi.org/10.1089/ jwh.2020.8882. Accessed October 20, 2021.
4. Niu L, Virani S, Bilheem S, Sriplung H. The effect of Pap smear screening on cervical cancer stage among southern Thai women. Sci Rep. 2019;9. https://doi.org/10.1038/s41598-019-52607-6. Accessed October 10, 2021.
5. Cervical Cancer: Screening. U.S. Preventive Services Task Force. August 21, 2018. Accessed October 10, 2021. https://www.uspreventiveservicestaskforce.org/uspstf/document/evidence-summary/cervical-cancer-screening.
6. Lei J, Ploner A, Elfström K M, et al. HPV vaccination and the risk of invasive cervical cancer. The New England Journal of Medicine. 2020;383(14):1340–1348. https://doi.org/10.1056/nejmoa1917338. Accessed October 12, 2021.
7. DeGroff A, Miller J, Sharma K, et al. COVID-19 impact on screening test volume through the National Breast and Cervical Cancer early detection program, January–June 2020, in the United States. Preventive Medicine. 2021;151. https://doi.org/10.1016/j.ypmed.2021.106559. Accessed October 10, 2021.
8. The American Cancer Society Guidelines for the Prevention and Early Detection of Cervical Cancer. American Cancer Society. April 22, 2021. Accessed October 12, 2021. https://www.cancer.org/cancer/cervical-cancer/detection-diagnosis-staging/cervical-cancer-screening-guidelines.html.
9. Fontham E T, Wolf A M, Church T R, et al. Cervical cancer screening for individuals at average risk: 2020 Guideline update from the American Cancer Society. A Cancer Journal for Clinicians. 2020;70(5): 321-346. https://acsjournals.onlinelibrary.wiley.com/doi/full/10.3322/caac.21628. Accessed October 10, 2021.
10. Curry S J, Krist A H, Owens D K, et al. Screening for cervical cancer. JAMA. 2018;320(7):674. https://doi.org/10.1001/jama.2018.10897. Accessed October 10, 2021.
11. Demarco M, Hyun N, Carter-Pokras O, et al. A study of type-specific hpv natural history and implications for contemporary cervical cancer screening programs. EClinicalMedicine. 2020;(22). https://doi.org/10.1016/j.eclinm.2020.100293. Accessed October 10, 2021.
12. Sawaya G F, Smith-McCune K, Kuppermann M. Cervical cancer screening: More choices in 2019. JAMA. 2019;321(20):2018-2019. https://doi.org/10.1001/jama.2019.4595. Accessed October 12, 2021.
13. Bedell S L, Goldstein L S, Goldstein, A R, Goldstein A T. Cervical Cancer Screening: Past, Present, and Future. Sexual Medicine Reviews. 2020;8(1):28-37. https://doi.org/10.1016/j.sxmr.2019.09.005. Accessed October 12, 2021.
14. Cholkeri-Singh A, Lavin P T, Olson C G, Papagiannakis E, Weinberg L. Digital colposcopy with dynamic spectral imaging for detection of cervical intraepithelial neoplasia 2+ in low-grade referrals: The IMPROVE-COLPO study. Journal of Lower Genital Tract Disease. 2018;(22):21-26. https://doi.org/10.1097/LGT.0000000000000353. Accessed October 20, 2021.
15. Moher D, Liberati A, Tetzlaff J, Altman D G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine, 2019;6(7). https://doi.org/10.1371/journal.pmed.1000097. Accessed October 2, 2021.
16. Hull R, Mbele M, Makhafola T, et al. Cervical cancer in low and middle income countries (Review). Oncology Letters. 2020;20(3):2058-2074. https://doi.org/10.3892/ol.2020.11754. Accessed October 8, 2021.
17. Morisada T, Saika K, Saito E, Kono K, Saito H, Aoki D. Population-based cohort study assessing the efficacy of cervical cytology (Pap smear) and human papillomavirus (HPV) testing as modalities for cervical cancer screening, Japanese Journal of Clinical Oncology. 2018;48(5):495–498. https://doi-org.ezproxy.csusm.edu/10.1093/jjco/hyy025. Accessed October 10, 2021.
18. Wentzensen N. ACS’s Updated Cervical Cancer Screening Guidelines Explained. National Cancer Institute. September 18, 2020. https://www.cancer.gov/news-events/cancer-currents-blog/2020/cervical-cancer-screening-hpv-test-guideline. Accessed October 10, 2021.
19. Nishimura H, Yeh P T, Oguntade H, Kennedy C E, Narasimhan M. Hpv self-sampling for cervical cancer screening: A systematic review of values and preferences. BMJ Global Health, 2021;6(5). https://doi.org/10.1136/bmjgh-2020-00374. Accessed October 10, 2021.
20. Yeh P T, Kennedy C E, Vuyst H, Narasimhan M. Self-sampling for human papillomavirus (hpv) testing: A systematic review and meta-analysis. BMJ Global Health. 2019;4(3). https://doi.org/10.1136/bmjgh-2018-001351. Accessed October 10, 2021.
21. Norenhag J, Du J, Olovsson M, Verstraelen H, Engstrand L, Brusselaers N. The vaginal microbiota, human papillomavirus and cervical dysplasia: a systematic review and network meta-analysis. BJOG An International Journal of Obstetrics and Gynecology. 2020;127(20):171-180. https://obgyn.onlinelibrary.wiley.com/doi/abs/10.1111/1471-0528.15854. Accessed October 10, 2021.
Marisa M. Walker earned an MPH with a concentration in Health Promotion and Education at California State University San Marcos. Her research areas include cervical cancer, HPV, vaccine hesitancy, women’s health, and health disparities.
Emily R. Bower earned an MPH with a concentration in Health Promotion and Education at California State University San Marcos. She is an Infection Preventionist Nurse with 9 years of diverse health care experience. Her research areas include infection prevention and control and women’s health.
Dr. Emmanuel Iyiegbuniwe is an Associate Professor of Public Health at California State University San Marcos (CSUSM) and has over 28 years of academic, administrative, and consulting experience. He earned both the MSPH and PhD degrees in Environmental and Occupational Health Sciences from University of Illinois at Chicago and MBA from Western Kentucky University. He served as the inaugural Director for the MPH program at CSUSM with concentrations in Global Health and Health Promotion and Education. He teaches several public health courses and conducts research and creative activities on issues related to social, environmental, and occupational determinants of health.
HPHR.org was designed by ComputerAlly.com.
Visit HPHR’s publisher, the Boston Congress of Public Health (BCPH).
Email communications@bcph.org for more information.

Click below to make a tax-deductible donation supporting the educational initiatives of the Boston Congress of Public Health, publisher of HPHR Journal.![]()