Hill M, Tremont J, McCraw M, Spach N, Berry A. Maternal mortality in the United States: a focus on health disparities. HPHR. 2021;34,
DOI:10.54111/0001/HH19
Maternal mortality is an important health problem with widely accepted international support for risk reduction. In contrast to other developed countries, maternal mortality rates in the United States have increased in recent years, resulting in a mounting public health crisis. Significant racial disparities in maternal mortality rates exist, particularly among Black and American Indian/Alaska Native women. Data demonstrate that social determinants of health – such as limited education, low income, rural residence and other environmental factors – place women at higher risk of suffering severe morbidity or mortality in the peripartum period. These determinants, compounded by structural racism, act as upstream drivers of maternal mortality. Greater access to care and improved quality of care are critical to reducing disparities. Policy initiatives to expand Medicaid, improve surveillance and mortality review committees, and identify barriers to quality care are necessary to achieve maternal health equity.
Since reaching peak levels in the early 20th century, Maternal Mortality Rates (MMR) in the United States (U.S.) have declined by 99%.1 Despite medical advances, maternal mortality rates in the U.S. stopped declining in the 1980s, and are currently on the rise.2 In 1986, the Centers for Disease Control and Prevention (CDC) in the U.S. founded the Pregnancy Mortality Surveillance System (PMSS) in order to conduct annual surveillance of trends in maternal mortality. Since implementation of the PMSS, the number of reported pregnancy-associated deaths have more than doubled in the U.S. from 1987 to 2016 (7.2 deaths to 17.3 per 100,000 live births).2 The CDC defines pregnancy-associated deaths as “the death of a woman while pregnant or within 1 year of the end of a pregnancy – regardless of the outcome, duration or site of the pregnancy – from any cause related to or aggravated by the pregnancy or its management, but not from accidental or incidental causes.”2
Maternal mortality is on the rise in the U.S., despite the spending of 16.9% of the U.S. GDP on healthcare in 2018; this stands in stark contrast to the consistent decline in maternal mortality in other similarly developed nations, which spend a smaller percentage of their GDP on healthcare.3 The United States is the only wealthy country in the world whose mothers are dying at a higher rate than 25 years ago.4 Rising maternal mortality rates in the U.S. have not been equal across all racial groups, with Black and American Indian/Alaskan Native (AI/AN) women most at risk.2 Importantly, the most frequent causes of death varied by race, and two-thirds of all the deaths were considered preventable.5,6
Recent data demonstrate that race, limited education, low income, and rural residence place women at higher risk of suffering severe morbidity or mortality in the peripartum period; thus, these factors are upstream drivers of mortality that warrant investment of resources.7–9 Maternal mortality has detrimental effects on surviving infants and their families,10,11 and can increase financial hardship as well as healthcare cost.12 This paper aims to comprehensively summarize the determinants of maternal mortality in the U.S., provide a model for understanding the complex contributing factors (Figure 1), and outline priorities for future intervention.
The Maternal Mortality Review Committee (MMRC) 2019 report findings showed that almost 75% of the 454 pregnancy-related deaths analyzed were due to cardiovascular conditions, hemorrhage, infection, embolism, cardiomyopathy, mental health conditions, and preeclampsia.6 For Black women, the top two causes – each accounting for 13.9% of deaths – were cardiomyopathy and cardiovascular conditions (including coronary artery disease, pulmonary hypertension, valvular disease, vascular aneurysm, and hypertensive cardiovascular disease).6 The top cause of maternal mortality for white women was mental health conditions (including suicide, overdose/poisoning and unintentional injuries determined by the MMRC to be related to a mental health condition), accounting for 14.9% of deaths, and cardiovascular conditions and hemorrhage, each accounting for 13.4%.6 The proportion of pregnancies complicated by pre-existing heart disease increased by 25% between 2003 and 2012, and the prevalence of cardiomyopathy and pulmonary hypertension have also increased significantly.13
Multiple genetic and epigenetic features have been identified in association with preeclampsia, as well as with obesity, diabetes, addiction and postpartum depression.14–17 Several studies have demonstrated an increased risk for maternal mortality, as well as severe maternal morbidity, for women with above-normal BMI.18–20 Inherited disorders that increase blood clotting, such as Factor V Leiden, may decrease the risk of postpartum hemorrhage and raise the risk of embolism.21 In the case of preeclampsia, family history and high blood pressure are known risk factors, as well as paternal factors including age of the father and paternal family history of cardiovascular diseases.22
Overall, biologic and genetic determinants play a significant role in maternal mortality (Table 1). While the top causes of pregnancy-related deaths differ by racial and ethnic groups, the incidence of cardiovascular disease is on the rise and affecting an increasing percentage of all pregnancies. However, a genetic risk factor alone does not cause disease, and interaction with the environment must be considered.
The delay of one’s initial prenatal visit and infrequent prenatal care are closely related to increased rates of maternal mortality. Black, Hispanic and AI/AN women are at an increased risk of delayed initiation of prenatal care compared to white women.23,24 Retrospective reviews of maternal mortality data have shown that, among women who died of pregnancy-related causes, Black and Hispanic women were more likely to have initiated prenatal care after the first trimester.24 One factor that heavily influences initiation and frequency of prenatal care in the Hispanic population is documentation status,25 with women who are undocumented having significantly lower rates of adequate prenatal care even after the expansion of Medicaid.26
In addition, inability to access primary care and lower utilization rates can impact the overall health of women, which can lead to poor management of chronic diseases like hypertension, diabetes, and obesity.27 Black women have higher rates of hypertension than their white counterparts and their pregnancies are more likely to be complicated by hypertensive disorders.23 Hispanic women and Asian/Pacific Islander women have higher rates of pre-pregnancy diabetes, which places them at higher risk for adverse pregnancy outcomes.23
Interpersonal relationships also have a significant impact on pregnancy-related maternal outcomes, including maternal mortality. Within the home, about 4-8% of pregnant women experience intimate partner violence, which significantly increases the risk of maternal mortality.28 Compared to other racial and ethnic groups, Black and AI/AN women have the highest risk of intimate partner violence, and the risk of pregnancy-related homicide in Black women is 3-7 times higher than in white women.28 In addition, in a qualitative thematic analysis, how women perceive their social status and how they view the significance of their marital relationship, among other factors, contributed to how women viewed maternal morbidity and their mental and emotional health.29
Altogether, behavioral and psychosocial factors create significant obstacles to accessing primary care for Black, Hispanic, AI/AN and undocumented women (Table 1). These challenges can lead to poor management of chronic diseases and delayed initiation of prenatal care, both of which put these populations at higher risk for maternal mortality.
Although sanitation standards in the U.S. and other developed nations are higher than in developing nations, environmental contributors to many leading causes of maternal mortality in the U.S. should not be discounted. A review of ecological studies found that variables related to sanitation, nutrition, and education may be stronger predictors of health status than economic indicators.30
Research shows that access to improved sanitation and safe water supply are both inversely correlated to MMR,31 and more than two million people in the United States live without running water and basic indoor plumbing.32 While the majority of Americans benefit from infrastructure that ensures access to safe, piped, potable water, low-income people living in rural areas, indigenous people and immigrants are those who suffer the most from lack of access to clean water.
Rural residence in the U.S. is also associated with higher rates of death for all causes compared to urban or suburban residence, and this holds true for causes of maternal mortality.33 Patients living in rural areas have to travel farther to access specialty physician and nursing services, and rural hospitals may not always have readily available life-saving clinical services such as large-volume blood transfusions.33 Less than half of rural counties have a physician trained in obstetrics and gynecology, and rural mothers are three to four times as likely to die from a pregnancy-related cause.34 Additionally, rural residents face unique challenges related to transportation, housing, economic resources, and food security.33
Food insecurity and maternal nutrient depletion both contribute to adverse pregnancy outcomes.35 Food insecurity is a significant issue for economically disenfranchised individuals in the U.S., with 14% of the U.S. population – over 40 million individuals – experiencing food insecurity.36 Pregnant people are particularly susceptible to environmental risks due to increased nutritional and metabolic demands associated with pregnancy.35 For example, pregnant individuals experiencing food insecurity may be at a greater risk for iron deficiency anemia, which is associated with increased risk of maternal mortality.37
Another environmental factor that is disproportionately felt by those living in poverty is the burden of air pollution, which may exacerbate clinical comorbidities and lead to increased risk for maternal mortality. Women identifying as Black, Hispanic, and Asian/Pacific Islander are twice as likely to live in an area of higher air pollution compared to their white counterparts.38 This concern is not unique for air quality; communities that are poor and non-white disproportionately face the burden of all environmental risk in the United States.39
In summary, environmental factors such as sanitation, regional availability of resources, nutrition and air pollution are all important predictors of health status (Table 1). Expectant mothers are particularly susceptible to environmental risks due to increased nutritional and metabolic demands. The burden of environmental risk disproportionately falls to the most vulnerable communities; therefore, these disparities should be acknowledged and addressed when managing pregnancies and planning maternal health interventions.
Key risk factors for pregnancy-related mortality include advanced maternal age, race, and socioeconomic status (SES). Among all racial groups, Black and AI/AN women die from pregnancy related causes at approximately three times the rate of non-Hispanic white, Asian, and Hispanic women.40 The national pregnancy-related mortality rate (PRMR) is 17.3 per 100,000 live births, but for Black and AI/AN women the PRMR is 42 and 28 per 100,000 live births, respectively.2 Many complex influences contribute to racial, ethnic and socioeconomic disparities, including social, political and economic forces.40
Mechanisms that contribute to racial disparities in maternal mortality are complex and multifactorial. Racism contributes to chronic stress, especially for Black women, which leads to increased risk of adverse pregnancy-related events.41 Research has found that Black women self-report higher perceived stress and more depressive symptoms regardless of income compared to their white counterparts.41 Black college-educated women have worse maternal health outcomes than white women who have dropped out of high school.42 Several prominent researchers have hypothesized that Black women may experience physical deterioration and aging at a more accelerated rate because of the constant exposure to the daily stress of racism, including the stress of discrimination, poverty, and unsafe living conditions.43
Other research has focused on the role of provider bias, resulting in a lower quality of care for Black women.44 One qualitative study on prenatal care experiences among Black women found that Black expectant mothers reported concerns that providers held negative assumptions about them and cited barriers in cultural sensitivity and quality of care.45 It is imperative to consider the ways in which racialized poverty contributes to increased risk of adverse maternal outcomes.
Additionally, an expanding body of evidence has sought to characterize the ways in which social, political, and economic structures contribute to racial disparities in maternal health outcomes.46 The link between poverty and poor health outcomes is well established in the literature with lower SES groups having a higher risk of mortality.47 Black and AI/AN women are disproportionately impoverished compared to other racial demographic groups,46 and, unsurprisingly, experience higher rates of maternal mortality. Women living in poverty are disproportionately under- or uninsured and generally have worse access to healthcare services, including prenatal care, compared to women with higher incomes.48 Additionally, Black women are more likely than white women to receive obstetric care at a hospital that provides lower quality of care (including fewer specialist services) compared to white women, regardless of access to care.49
Moreover, Black and AI/AN women of low SES have worse access to resources that promote health, such as adequate nutrition and safe housing, compared to their white counterparts.50 This drives the higher rates of chronic diseases in these populations, including hypertension, diabetes, and cardiovascular disease.50
Socioeconomic disparities among Black and AI/AN communities in the United States have been influenced by centuries of genocide, slavery and systemic oppression. In addition, wealth disparities have been perpetuated through centuries of racist policies that promote exclusion, segregation, displacement and destabilization of communities of color.51 Wealth determines a family’s – and, more broadly, a community’s – ability to access many critical social goods and resources, such as education, healthcare, transportation, and clean and safe environmental conditions. Neighborhood violence and general exposure to trauma and stress are associated with adverse maternal outcomes,52 and Black women are four times more likely to live in a neighborhood with high poverty and crime rates compared to white women.53
Medicaid expansion is one example of how policy decisions contribute to maternal health disparities of Black and AI/AN women. With the enactment of the Patient Protection and Affordable Care Act (ACA) in 2010, Medicaid eligibility was expanded to non-elderly adults up to 138% of the federal poverty level (FPL), and income-related subsidies were offered to support the purchase of health insurance through the federal or state insurance marketplace for individuals or families with incomes between 100% and 400% FPL.54 However, in 2012, the U.S. Supreme Court determined that Medicaid expansion could not be federally mandated, and the decision to expand eligibility was left to individual states.54 As of March 29, 2021, there are still 12 states that have not adopted Medicaid expansion, half of which are historically conservative states in the south: Florida, Georgia, Mississippi, North Carolina, South Carolina, and Texas.55 Notably, the percentage of uninsured adults in 2019 was nearly two times higher in non-expansion states compared to states that opted to expand eligibility (15.5% vs 8.3%),56 and many individuals falling into the insurance coverage gap are women of reproductive age.57 Since many southern states have opted to not expand Medicaid, and Black women are more likely to reside in the south, many Black women fall into the coverage gap and have remained under- or uninsured.58,59 Insurance status has deleterious effects on access not only to prenatal care, but also to general healthcare services, including preconception counseling, family planning and basic primary care services.
Overall, social, political and economic factors that contribute to racial disparities in maternal mortality are complex, multifactorial, and pervasive (Table 1). Centuries of systemic racism have perpetuated the destabilization of communities of color and contributed to the higher burden of stress, higher rate of poverty, and lower quality of care experienced by Black mothers. The pregnancy-related mortality rate is significantly higher for Black and AI/AN women, warranting further investigation and targeted interventions.
Table 1: Key statistics and key takeaways for each determinant of maternal mortality
Determinant | Key Statistics | Key Takeaways |
Biological and genetic determinants | – Almost 75% of PRD in 2019 were due to CV conditions, hemorrhage, infection, embolism, cardiomyopathy, mental health conditions, and preeclampsia. – The top two causes of PRD for Black women were cardiomyopathy and CV conditions (13.9% each). – The top three causes of PRD for white women were mental health conditions (14.9%), CV conditions (13.4%) and hemorrhage (13.4%). | – The proportion of pregnancies complicated by pre-existing heart disease increased by 25% between 2003 and 2012, and CV conditions account for a large and increasing percentage of PRD. – Genetic risk factors must be considered in the context of one’s environment. |
Behavioral and psychosocial factors | – Black, Hispanic and AI/AN women are at an increased risk of delayed initiation of prenatal care compared to white women. – Women who are undocumented have significantly lower rates of adequate prenatal care. – About 4-8% of pregnant women experience IPV, which significantly increases the risk of maternal mortality. Black and AI/AN women experience the highest rates of IPV. | – Psychosocial factors may create obstacles to seeking and accessing care, leading to poor management of chronic diseases. – Black women have higher rates of hypertension, and Hispanic women and Asian/Pacific Islander women have higher rates of pre-pregnancy diabetes, all of which increase the risk of maternal mortality for these populations. |
Environmental factors | – Improved sanitation and safe water supply are both inversely correlated to MMR, and more than two million people in the U.S. live without running water and basic indoor plumbing. – Rural residence is also associated with higher rates of PRD – Less than half of rural counties in the U.S. have a trained Ob/Gyn, and rural mothers are three to four times as likely to die from PRD. – Pregnant women who are part of the 14% of the U.S. population experiencing food insecurity are at increased risk for malnourishment. | – Variables related to sanitation and nutrition may be stronger predictors of health status than economic indicators and can significantly affect pregnancy outcomes. – Pregnant people are particularly susceptible to environmental risks due to increased nutritional and metabolic demands associated with pregnancy. – Communities that are poor and non-white disproportionately face the burden of all environmental risk in the U.S. |
Social, political, and economic structure | – Black and AI/AN women have three times the rate of PRD compared to non-Hispanic white, Asian, and Hispanic women. – The national PRMR is 17.3 per 100,000 live births, but for Black and AI/AN women the PRMR is 42 and 28 per 100,000 live births, respectively. – Black college-educated women have worse maternal health outcomes than white women who dropped out of high school. – Black women self-report higher perceived stress and more depressive symptoms regardless of income compared to their white counterparts. – Provider bias may result in a lower quality of care for Black women, and Black women are more likely than white women to receive obstetric care at a hospital that provides lower quality of care, despite access. | – Social, political and economic factors that contribute to racial disparities in maternal mortality are complex, multifactorial, and pervasive. – Socioeconomic disparities among Black and AI/AN communities in the U.S. have been influenced by centuries of genocide, slavery and systemic oppression. – Medicaid non-expansion in mostly southern states has left many Black women under- or uninsured, which has deleterious effects on access to prenatal and general primary care. |
PRD = Pregnancy-related death. CV = Cardiovascular. IPV = Intimate partner violence. AI/AN = American Indian/Alaska Native. MMR = Maternal Mortality Rate. Ob/Gyn = Obstetrician/Gynecologist. PRMR = Pregnancy-related mortality rate. | ||
The factors contributing to maternal mortality in the United States are complex and interconnected. Figure 1 illustrates a causal-loop model for maternal mortality, including select upstream contributors with a focus on limited access to care. One intervention that has increased access to care for underserved populations is the funding of Federally Qualified Health Centers (FQHCs). The criteria for FQHCs was established by Congress in 1989 and includes requirements that FQHCs must be located in underserved areas and provide services regardless of the patient’s ability to pay, among other requirements.60,61 While FQHCs are helpful, there is still a large population of underserved people living in areas without access to a FQHC or the equivalent, leaving them without options for care.60Substantially expanding the reach of FQHCs would likely improve maternal outcomes significantly, not only through increased access to prenatal care, but also through increased access to care over an individual’s lifespan, leading to improved baseline health status.
Additionally, there is currently a shortage of primary care clinicians in the United States, including a critical lack of providers trained in maternal health, especially in rural areas of the country.62 This shortage is driven by a number of factors including the large percentage of practicing physicians who are approaching retirement; the high cost of medical training that deters graduates away from the lower compensation associated with primary care; and the arbitrary cap on Medicare funding for residency positions at 1996 levels that has limited the opening of new residency positions despite increasing medical school enrollment.63 The causal loop diagram in Figure 1 illustrates how limited access to care, such as the dearth of obstetricians and gynecologists in rural counties, may contribute to the higher risk of death with rural pregnancies.34 Policy makers must address the physician shortage to reduce these regional disparities by eliminating the Medicare cap, directing more funds for graduate medical education, and encouraging the establishment of new residency positions. Further investments would be required to train physicians and advanced practice providers (e.g. physician assistants, and nurse practitioners) to provide primary care at FQHCs and health centers in rural communities.64–66 It may be a challenge to convince graduates with a burden of educational debt to go into these types of primary care practices, but one feasible strategy to overcome that obstacle is encouraging loan forgiveness programs for graduates who choose primary care specialties or commit to practicing in a rural community.67
Evidence is mounting that Black women are at disproportionate risk of receiving poor quality maternity care. One potential avenue to address quality disparities is Medicaid expansion. A cross-sectional study of federally-funded health centers after Medicaid expansion found that health centers in expansion states had better performance in multiple quality measures compared to centers in non-expansion states, including that expansion state health centers cared for more patients, had more patient visits, and had a higher proportion of their patients covered by Medicaid.54 Medicaid expansion states also outperformed non-expansion states in multiple clinical outcome measures related to primary care utilization, including control of blood pressure and diabetes, which affect pre-pregnancy health status, and early initiation of prenatal care.54 Expanding a woman’s ability to access high-quality healthcare both before and during pregnancy is crucial to addressing maternal mortality. Medicaid expansion is one policy approach to increase insurance coverage, increase rates of prenatal care and improve pre-pregnancy health status, therefore lowering the risk of maternal mortality for vulnerable populations (Figure 1).
Although it is clear that racism increases maternal mortality risk for women of color, even in the presence of other potentially protective factors such as education, insurance and income, it is less clear how health systems, which cannot by themselves eliminate systemic racism, can intervene to protect from the effects of racism. One area for intervention might involve addressing the role of community and social support in mitigating the effects of chronic stress during pregnancy (Figure 1). Research supports that the integration of community health workers, doulas and other social support groups into routine pregnancy management can empower and educate patients, provide tools for coping with stress, minimize health disparities and possibly reduce maternal mortality.68–70 The involvement of pregnancy doulas, who provide individualized support before, during, and after birth, has been shown repeatedly to improve both maternal and infant outcomes related to premature delivery, obstetric complications, and initiation of breastfeeding.71–73 Community-based support interventions, while not a cure for racism, have the potential to interrupt the connection between racism and maternal mortality and should be a priority for federal and state policy makers.
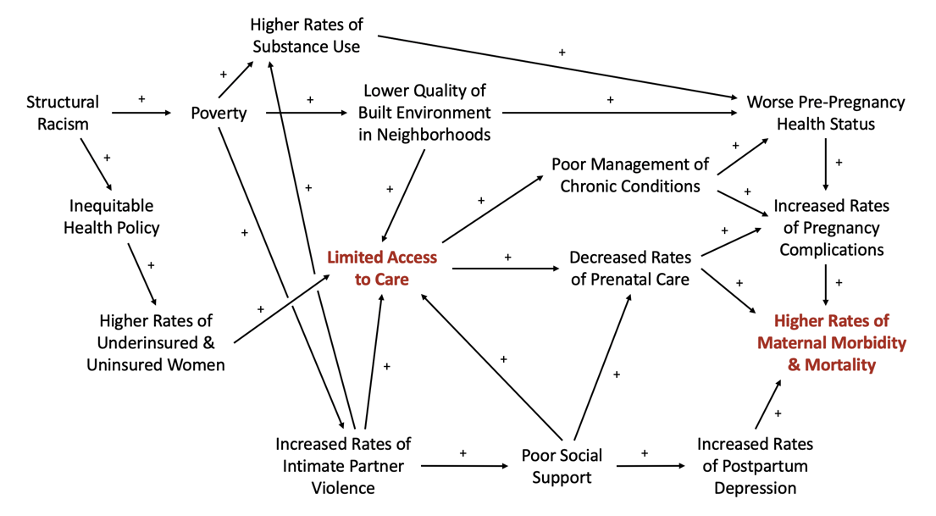
Figure 1. Causal loop diagram depicting select determinants of maternal mortality in the U.S.
Our analysis has several limitations. While this paper delineates why maternal mortality is on the rise in the U.S., it remains a challenge to assess the true extent of maternal mortality due to differences in reporting and availability of data. Maternal mortality is still such a rare occurrence in the U.S. that it is not often observed in any randomized, controlled trials; thus, it is difficult to draw conclusions on limited empirical evidence.74 In addition, changes in the methodology and coding of collecting information regarding “pregnancy-related” deaths may result in misclassification and underreporting of maternal morbidity.75,76 While the CDC defines pregnancy-related deaths as occurring any time from conception to one year after delivery, this time frame for inclusion differs vastly between studies. Coding errors or differences in how hospitals record data for pregnancy-related deaths may subsequently lead to errors in rate and risk estimations.77 Different hospitals may also have code variations based on severity of pregnancy-related complications, which limits tracking of data uniformly across systems.77
Furthermore, place and cause of death is not absolute; some patients will die in the hospital despite their death being caused by events prior to admission, and some patients may die outside of the hospital despite death being attributable to medical errors.78 Data are often derived from vital statistics and administrative databases (such as hospital discharge information or death certificates). These sources may be incomplete or inaccurate, which challenges the ability to precisely assess maternal deaths.76 Some complications may not present until after hospital discharge, resulting in underreporting in studies exclusively using hospitalization data.77 Lastly, data with analyses based on race are subject to inaccuracies. The incomplete and imprecise reporting of data on maternal deaths and morbidity is especially prominent as it relates to self-identified racial and ethnic categories. Racial categories are social constructs, and one’s racial identity may evolve over time, making it difficult to generalize more broadly.79
Health inequity is a critical driver of maternal mortality in the United States. Upstream factors include access to and quality of care, racial and ethnic disparities, and pre-pregnancy health status. Support for public health policy that encourages women to seek preventative screenings, low-cost medications, and disease education in universally understandable terms is necessary. However, the responsibility to combat increasing rates of maternal mortality cannot fall to those patients most affected by it – namely communities with higher baseline risk due to racism and other complex medical, environmental and social factors. Federal, state and local policy makers must partner with clinicians and communities to guarantee protections for vulnerable patients. Possible interventions include greater investment in Federally Qualified Health Centers, expansion of Medicaid to all states in the U.S., and establishment of pregnancy support networks and doula programs. These changes would benefit not only individual women, but also families and society as a whole.
Thank you to Dr. Jon Hussey for his unwavering support of our efforts to explore this salient and essential public health matter.
Melissa B. Hill is a rising 4th year medical student at the Icahn School of Medicine at Mount Sinai in New York City. She is currently earning her Master of Public Health degree from the University of North Carolina Gillings School of Global Public Health in Chapel Hill, NC. Her interests include full-spectrum reproductive healthcare, addressing health disparities in pregnancy, and medical education.
Dr. Jaclyn N. Portelli Tremont is a resident in General Surgery at the University of North Carolina. Her research interests involve health disparities, improving health care access, and addressing social determinants of health.
Michelle C. McCraw is a fourth year medical student at the University of Florida College of Medicine in Gainesville, FL. She is currently earning her Master of Public Health degree from the University of North Carolina Gillings School of Global Public Health in Chapel Hill, NC. Her professional interests include maternal health, immigrant health, and global public health.
Natalie Christine Sprach is an MD/MPH candidate at the University of North Carolina School of Medicine and Gillings School of Global Public Health. She applying into obstetrics and gynecology for residency. Her interests are health policy, family planning and medical education.
Anne Maria Berry, MD is a family physician who has worked for 5 years at a Federally-Qualified Health Center in Clendenin, WV, and 3 years at a small hospital outside Mwanza, Tanzania. She then returned to residency to complete training in the specialty of Preventive Medicine at UNC. Her research and clinical interests include maternity care and maternal health disparities, community-based participatory research and health policy.
HPHR.org was designed by ComputerAlly.com.
Visit HPHR’s publisher, the Boston Congress of Public Health (BCPH).
Email communications@bcph.org for more information.

Click below to make a tax-deductible donation supporting the educational initiatives of the Boston Congress of Public Health, publisher of HPHR Journal.![]()