Starble E. Implications of robotic surgery. Harvard Public Health Review. Fall 2018;14. DOI:10.54111/0001/N4
The interest that I have in robotic surgery stems from the discrepancies between literature at the time that my current hospital purchased their system and the aggressive marketing campaign from the parent company. In urology, the robot is marketed as “nerve-sparing” during prostatectomies and in cardiac it allowed more dexterity or finer motor control. The bulk of the concerns center around issues such as system resetting; electrosurgical arch; increased surgical time; prolonged carbon dioxide insufflation; unnecessarily extended amounts of time under anesthesia; and extremely high costs to purchase and maintain the system on site.
A cost-based analysis and a surgical time consideration of robotic versus laparoscopic and open procedures disprove the regularly cited benefits of using a robotic console over the trained surgeon hands for not only surgical risk but financial benefits to both the facility and patient. I gathered records of robotic, laparoscopic and open procedures, the time each takes, the financials for instrumentation and the legal issues or complication associated for each.
The robotic technique is a greater cost than any procedure comparable and at an increase of surgical and/or anesthetic time. The greater the anesthetic time, the more potential harm to patients. The greater the surgical time, the higher the risk from increased pneumoperitoneum CO2 insufflation on the cardiovascular and respiratory systems. The higher the cost ratio, the less benefit for the practicing facility. Legalities are a new issue to consider when the robot or the parent company is found at fault considering the health insurance agencies have taken a stand on compensation for only the diagnosis and not the techniques or technology utilized for that treatment.
Is robotic surgery as beneficial financially or in terms of convalescence to the patient, surgical team and facility as open or laparoscopic surgery?
The Da Vinci surgical robot has become the latest innovation for laparoscopic procedures by improving on visualization and increasing dexterity in surgery since its approval by the U.S. Food and Drug Administration in 2000. Laparoscopic surgery has been our standard of practice for the last 30 years with the first procedure being a laparoscopic cholecystectomy in 1987, and although there are the limitations of two-dimensional imaging and an impaired range of motion or ergonomics for optimal control over the instrumentation, this method proved to be less traumatic for the surgical patient. With technological advances in every other industry, it is difficult not to appreciate the added control and visualization that the robotic systems have delivered. By increasing the range of motion for instrumentation, adding 3D imaging and removing the natural human tremor with screen stabilization, the only obvious drawbacks become time and cost.
A basic Google search of available literature was performed using the terms “articles robotic vs. laparoscopic general surgery” with 445,000 results, “robotic vs. laparoscopic general surgery meta-analysis” with 379,000 results, showing a dramatic increase in concern over cost-based analysis in healthcare technology. Federal guidelines have risen the demands on quality while at the same time cut or decreased the reimbursements for procedures and hospital stay. Hospitals struggle to stay “cutting-edge” and at the same time, struggle with the budgeting to do so.
My background is as a surgical nurse for the last 15 years. I have my CNOR national certification and I am one of the advanced surgical clinicians on staff at Mount Auburn Hospital in Cambridge, Massachusetts. I am one of the clinical preceptors for the perioperative program of Regis nursing students for my hospital and have helped in training medical and physician assistant students in the operating room acclimations. My position on staff is as the clinical lead for general, vascular and bariatric surgeries, so the access that I have as a purchasing agent for hospital supplies and equipment has always made me curious as to whether there was a better or less costly alternative. I purchase much of supplies for our operating room and all specialty items for my services, giving me a unique window into the sales groups and purchasing powers that we can use to drive down costs. Working with purchasing groups, sales representatives and management teams, I have access to the financials on actual instrumentation costs and surgical times. I planned to use my hospital’s contracted rates of purchase as the standard or average for other facilities.
I coordinated the surgical side of conversions from Ethicon to Applied Medical to Covidien trocars for laparoscopic surgeries, cutting our costs for the supplies each time. I coordinated the conversion of hernia mesh from owned to consignment relieving our direct inventory costs surgically and all of our vascular grafts to strict consignment with Gore Propaten grafts, saving at least $1000 per patient. I also inventory least used stock and discontinue supplies that are no longer in use, discussing with the purchasing department and company for reimbursements. I built the bariatric practice from the ground up and worked with a new group of vascular surgeons to help build a vascular practice in conjunction with interventional radiology instituting new procedures, standards, and techniques to the hospital. In conjunction with the central supply department, I helped trim the weight of our instrumentation in the pans or kits to be under the guidelines for sterilization keeping our hospital ahead of the state and national expectations.
I learned to budget through my own experiences running construction crews on income property that I have purchased. I had to schedule and budget for a complete tear-down and renovations to each unit. Planning for plumbers, electricians, and flooring to work around the carpenters and plasterers allowed for a more continuous workflow and shorter downtime.
With healthcare spending one of today’s hot topics, hospital fiscal waste under evaluation and the ever-shrinking margins, I noticed that the extra spending on new equipment might not be the investment to pursue. One of the newer trends and latest purchases to my hospital was the Da Vinci robot from Intuitive Surgical. When I saw the initial purchase price, the cost per instrument and the commitment that the hospital had to show by not just sending people out for training, but designing a suite exclusive for this service, I immediately was shocked. With my surgical equipment knowledge and the budgeting/accounting experiences that I have learned, the amount of hospital waste in this one surgical service drew my immediate attention.
Despite the device being initially meant for cardiac surgery, recent years have witnessed a rapid growth in its application to abdominal surgeries by urology, gynecology, and general surgery. The interest that I have in robotic surgery stems from the discrepancies between literature at the time that my current hospital purchased their system. In urology, the robot is marketed as “nerve-sparing” during prostatectomies and in cardiac it allowed more dexterity or a finer motor control for delicate vessels. When researched, these were “half-truths.” The system does allow for more dexterity and finer motor control, but there are numerous accounts of the system resetting, electrosurgical arch and extremely high costs to purchase and maintain the system on site, this last being the most concerning. For the patient, an increased surgical time, prolonged carbon dioxide insufflation and unnecessarily extended amounts of time under anesthesia become added incidentals for consideration when there is very little difference between the length of hospital stay depending on the procedure. I believe that a cost-based analysis and a surgical time consideration of robotic versus laparoscopic and open procedures will disprove the regularly cited benefits of using a robotic console over the trained surgeon hands. Surgery is a specialty area for all medical providers. How will the usage of computer guidance affect the skill-set and training of the surgeons and the operative nursing staff?
The differences between the two types, robotic and laparoscopic, focus on technique. In laparoscopy, the instrumentation and field of view are opposite, up-down and left-right. For a robotic approach, the view can become the same as an open procedure where the surgeon’s hand moving to the right will move the robotic hand to the right. This can give the surgeon more of an “open” procedure sense of control. The next difference is in the instrumentation. The traditional laparoscopic instrumentation is considered to be more rigid and therefore the port sites are dictated by the angles needed for intended structures and the desired critical view. The robotic instrumentation provides an increased rotation and dexterity that allows for access to greater turning radius while at difficult angles without the need to change ports or add a new site. Robotic colectomies have been found to be both more time-consuming and expensive than the standard laparoscopic approach but that it resulted in faster recovery of bowel function, shorter hospital stay, less blood loss, less postoperative complications, and lower wound infection rates.
Robotic surgery has been a field of focus for approximately 30 years. NASA and the Ames Research Center were developing what would be known later as the beginnings of virtual reality software in the late 1980s. While looking at potential applications, the idea of using virtual reality for surgical procedures was investigated as a way to minimize the surgical incision sites, knowing the smaller incisions has less pain and faster healing. NASA and Ames partnered with the Stanford Research Institute to evaluate design and surgical preferences around 1990. The US Army became interested in the idea of telesurgery and the idea of “bringing the surgeon to the wounded soldier—through telepresence.”
With military funding, the goal became to provide surgical intervention for the wounded soldier in the field while the surgeon was at a M.A.S.H. unit some distance away from the engagement. Upon leaving the military, some of the engineers brought the technology to the public and formed commercial companies. Computer Motion developed the AESOP for voice-automated camera positioning and the ZEUS robotic system for instrumentation manipulation. Intuitive Surgical bought out Computer Motion, merged the two companies in 2003 and discontinued the ZEUS in favor of the Davinci, which was the higher-priced product and now had no competition.
The history of robotic surgical devices begins with the PUMA 560 in 1985 during a brain biopsy, then PROBOT in 1988 for prostatectomy and ROBODOC in 1992 for bone milling during hip replacement surgery, replacing 1983’s ARTHROBOT as a robotic surgical assistant. Beginning in the early 2000s and continuing through today, the primary robotic device in service has been the Da Vinci from Intuitive Surgical.
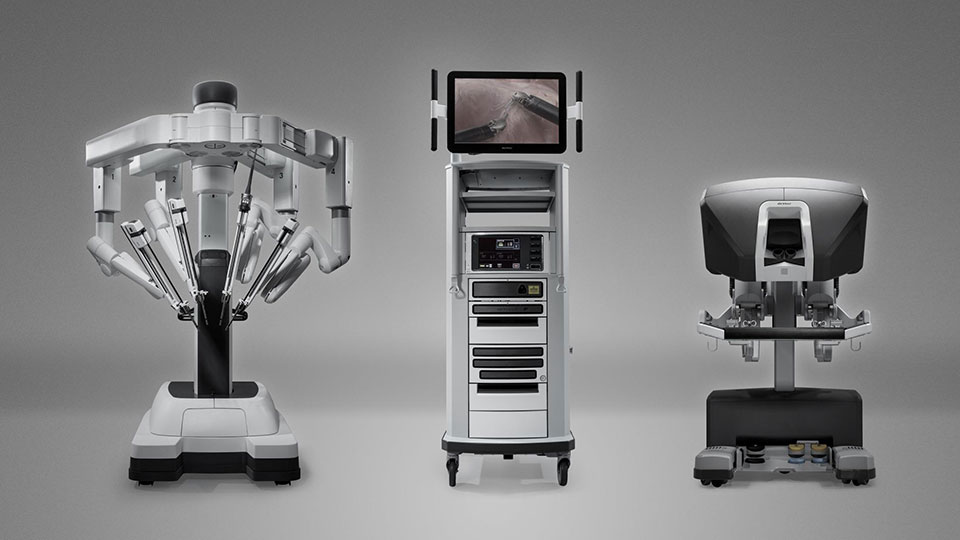
The Da Vinci Robot consists of three main pieces of equipment, from left to right;
The patient “side-cart” – This is the image most of us have. 4 arms that are connected to a base and maneuvered into position by staff at the beginning of each surgical procedure.
The vision system – The 3-D image generator, HD endoscope, and processing equipment.
The surgeon console – This is where the attending surgeon can sit comfortably and unsterile with hand, finger and foot controls while viewing a 3-D image.
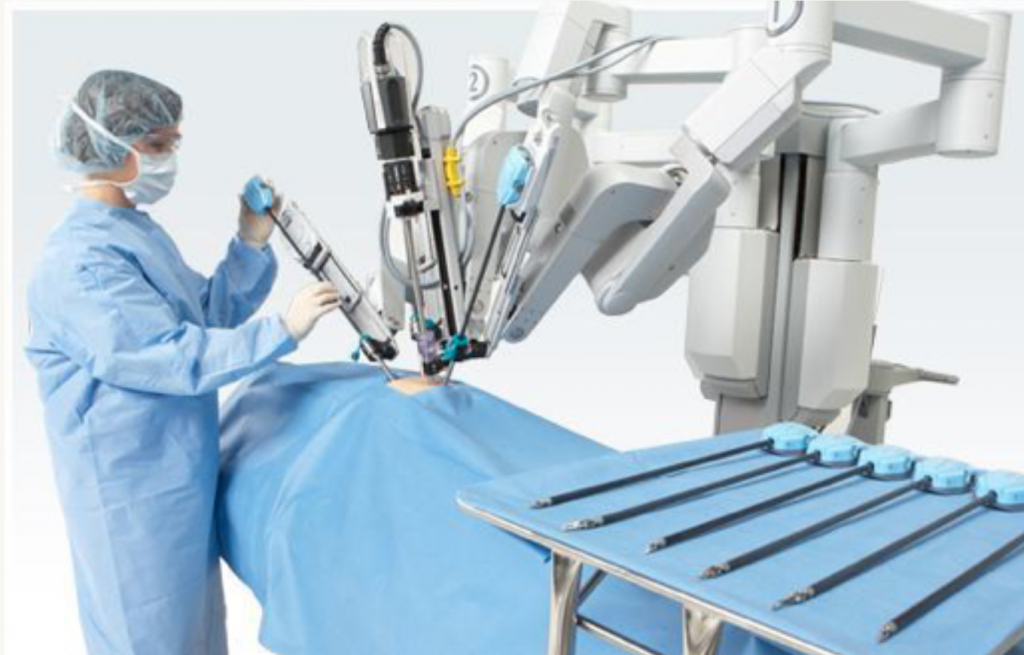
The other parts are the detachable instrument arms or “endowrists.” These are an operable joint consisting of wires and pulleys to provide 90 degrees of articulation with 7 degrees of freedom and are applied to various clamps, dissectors, and cutting tools.
There is always a drive to do better, or to “find that better way,” but is robotic surgery at a point where it is better than the current methods?
Ethically, the issue of informed consent brings up concerns with the “latest and greatest” for technology innovations. With the steep learning curves associated with new technologies and the extremely steep learning curve for robotics, surgeons may not be as forthcoming with the level of expertise they have or the added risks associated with robotic surgery. The example of a traditional open thyroidectomy being of a high success rate but leaving a noticeable scar across the patient’s neck versus the robotic approach, incision and scar will be further away leaving the patient less self-conscious is a desirable outcome. The concern is that there is no 20-year success data to compare against and the added robotic potential complications come in to play along with longer surgical times. There is also a conflict of interest when it comes to the design of the robot and/or techniques associated with learning. These have been designed under the supervision of or in congress with consultant surgeons. This means that they are receiving payments from the company and setting up preceptorship programs for teaching others in the field. There is a growing concern as to whether the consult surgeons have reasonably critiqued or evaluated the robot’s design, value or intended usage. It would be in the best interest of the facilities utilizing the robotic technology to develop new policies dedicated to the care and performance of the procedure along with insurance coverage in the event of failure of during any part of the procedure. Informed consent is another ethical concern. With the steep learning curve associated with robotic surgery, the pathway is longer for surgeons to acquire accreditations. In addition to prerequisite training and general surgical training needed to become an OR attending, the AAGL laparoscopic association for gynecological surgeons had released a set of guidelines and privileging requirements for robotic competencies:
Guideline 1: consists of the following six direct robotic training exercises:
Guideline 2: Because robotic surgical skills degrade substantially within weeks of inactivity in newly trained surgeons, the first proctored case should be performed no longer than 2 months after training has been completed. Otherwise, the training should be repeated.
Guideline 3: Surgeons who complete the recommended training pathway may be eligible for approval by the hospital MEC or appropriate governing hospital body to receive privileges to perform procedures designated as ‘‘Basic Robotic Surgery Cases.’’
Privileging Requirements for Basic Procedures
Another concerning observation is that of Intuitive Surgical providing grants for robotic fellowships. For the current fiscal year of 2017-2018, the funding per robotic fellow provided by the Da Vinci parent company is $62,500 for the areas of colorectal, general and thoracic surgery. These are the areas of least volume and where the focus for gains has been.
In Cardiothoracic, the main concerns are centered around anesthesia and surgical times. If there were an issue and the surgeon had to change to open from robotic, there would be a conversion time to overcome. The robot adds time to the procedure – cardiac surgery is already a 5-hour operation, and must now be increased by 1 hour to compensate for robotic technique. The anesthesia now has concerns over CO2 insufflation, increased intrathoracic pressure and decreased venous returns. External defibrillation pads might be in less effective placement due to the surgical field and inhibited by the increased CO2 in the cavity.
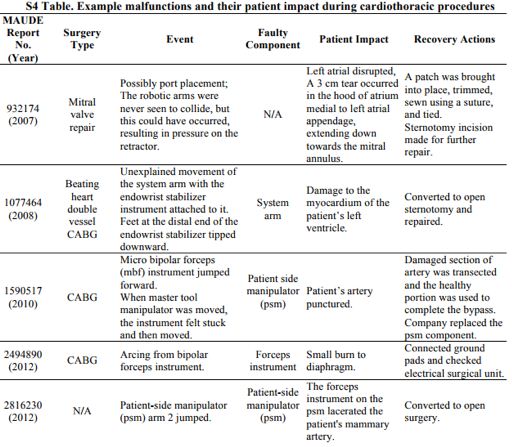
The FDA Manufacture Device User Experience is a voluntary reporting database designed to track trends in equipment that has agency approval. The FDA MAUDE review by Dr. Alemzadeh shows a brief view of the cardiac concerns reported. With lacerations and burns happening inside the chest cavity, the conversion to an open surgical approach for fast repair becomes a time constraint to consider.
With Gynecology, the study by Columbia University titled “Comparative Effectiveness of Robotically Assisted Compared with Laparoscopic Adnexal Surgery for Benign Gynecological Disease” was conducted between 2009 and 2012 and the data was collected from 87,514 women receiving surgical intervention for oophorectomy or cystectomy. Robotic-assisted oophorectomy increased from 3.5% to 15.0%, and robotically assisted cystectomy from 2.4% to 12.9%. The complication rate was 7.1% for robotically assisted and 6.0% for laparoscopic oophorectomy. Robotic-assisted oophorectomy was associated with a higher rate of intraoperative complications 3.4% versus 2.1%. The overall complication rate was 3.7% after robotically assisted versus 2.7% for laparoscopic cystectomy. The intraoperative complication rate was higher for robotically assisted cystectomy 2.0% vs. 0.9%. Compared to laparoscopy, robotically assisted oophorectomy was associated with an average total increase of $2,504 and robotically assisted cystectomy costs increased by an average of $3,310. In a study of over 264,000 hysterectomy patients performed over 441 hospitals found that robotics added an average of $2,000 per procedure.
In General Surgery, the studies are still new and ongoing, therefore, the statistical data is primarily meta-analyses.4 The overall results so far have been that for colorectal and primarily either right/hemicolectomies, left/sigmoid colectomies, and low anterior resections, that there is no significant difference in the hospitalization time or postoperative complications. In patients who underwent robotic right colon resections, the operative time was significantly longer from 34.21 to 70.43 minutes. Robotic approaches did result in less blood loss and a faster return of bowel function. No differences were found between robotic or laparoscopic technique for anastomotic leak, postoperative ileus or wound infection. The cost difference was significant at $19,231 vs $15,807 with robot providing a financial increase and increased operating times with no yet added benefit to general surgery.
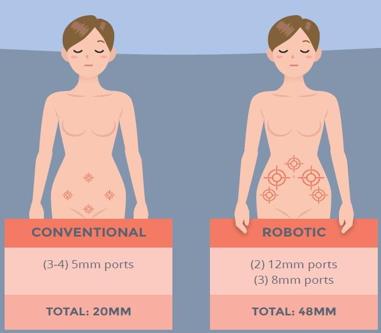
“For multi-port cholecystectomy performed by a seasoned laparoscopic surgeon, it is almost impossible to envision any way to improve any outcome measure. In essence, for multi-port cholecystectomy, robotics decreases the value of care by increasing costs without improving quality.” The American College of Surgeons article goes on to discuss charges for reimbursement by stating that the CPT billing code is the same 47562 for cholecystectomy whether it is performed laparoscopically or robotically. Once we get into inguinal hernia repair, the discussion becomes even harder to improve upon. The traditional laparoscopic repair of an inguinal hernia has 3 port sites, two 5mm, and one 12mm, where robotically there are two 8mm, a 5mm and a 12mm for a total of 4 port sites. At the time of this 2007-2009 chart review, the surgical time for the procedure was 1 hour and 13 minutes compared to 2 hours and 53 minutes. There is a steep learning curve, but the argument for one less surgical port site and 100 fewer minutes of surgical or anesthetic time is difficult to counter. Without teaching or complications, my surgeons average 30-60 minutes for laparoscopic repair of a unilateral inguinal hernia. The discussion of attempting robotic repairs are usually met with a question: how is this an improvement?
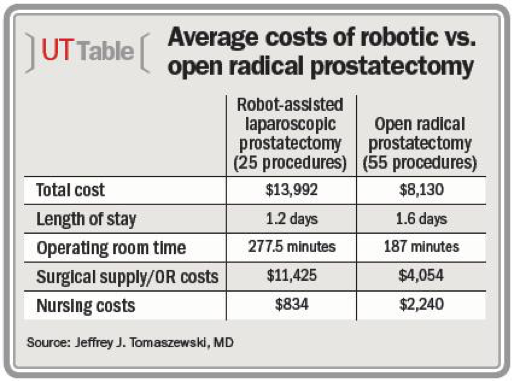
For Urology, prostatectomies are the study focus along with using open versus lap and robotic. The end results of continence and potency being virtually identical postoperatively, blood loss is significantly lower but transfusion rates did not change, the only difference medically was in the rates of positive margins at 15% for robotic, 27% laparoscopic and 35% for open radical prostatectomies. The cost difference was on average $487 less for open vs laparoscopic and increased $1726 robotically. In the “Robotic vs. Retropubic radical prostatectomy in prostate cancer:” meta-analysis paper, the review used 78 cases to determine that the surgical time was slightly higher at 40 minutes, but the hospital stay was shorter and recovery rates higher under robotic assistance. This was in response to other Urological articles suggesting the need for increased criteria and methodology due to the low quality of evidence for pursuing the robot-assisted prostatectomy approach as a viable option. In a chart review study by the University of Pittsburgh to determine the patient and hospital benefits for robotic versus open radical prostatectomy, Dr. Joel Nelson found the average length of stay for robotic prostatectomy was 1.2 days versus 1.6 days for open radical prostatectomy, showing no significant benefit. Robotically, prostatectomies require more surgical time at 277.5 minutes, compared to 187 minutes for open at a 48% increase, and cost 71% more per procedure. The total cost of 25 robotic procedures averaged $13,992 compared to $8,130 for 55 radical prostatectomies with surgical supplies and operating room costs $11,425 vs. $4,054, by more than $7,000. Nursing costs were significantly higher for open prostatectomy in this chart review as well, $2,240 compared to $834. The opposite side of the procedure is the total reimbursement for the hospital charges from insurances and patient costs were too similar to be cost effective at $10,000 to $11,000 per procedure. “The average OR supply costs for RALP are eight times greater than the supply costs associated with radical retropubic prostatectomy. There was no difference in cost for ancillary, cardiology, imaging, administrative, laboratory, and pharmacy costs.”
One of the other potentials to be addressed is the opportunity for “telesurgery.” This is when the operating room and machine are in one area and the conducting surgeon is in another. The concerns here are that:
This can become a useful tool in battlefield settings allowing soldiers to receive specialized surgical care that may not be available in the areas of deployment, but outside of scenarios like this, wouldn’t you want your surgeon in the same room as you or your family member?
“A surgeon actually does (his or…) her job better when they can compartmentalize their view of the human they are working on as a fellow person.” The thought here is that the surgeon can identify the patient as a person if they can see a body in front of them versus looking through the viewer and only seeing a small operative field. When training newer staff in surgery, this was always a topic we would try to impress upon them. Once the drapes are up and the incision is made, they only see the operative field and forget about the rest of the body. This is when someone may lean on the patient without realizing it or even balance their instruments on the patient’s leg. If you can’t see it, you sometimes forget what’s under there.
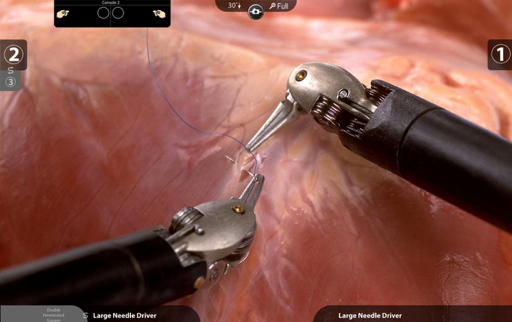
The detail is far superior to that of any other laparoscopic field. The image intensifier allows the operating surgeon to view and address each individual need with more precision and dexterity than prior techniques. The concern is that this is all that they are seeing. There is no tactile or extraneous notion that this is a person other than what is kept in mind while working.
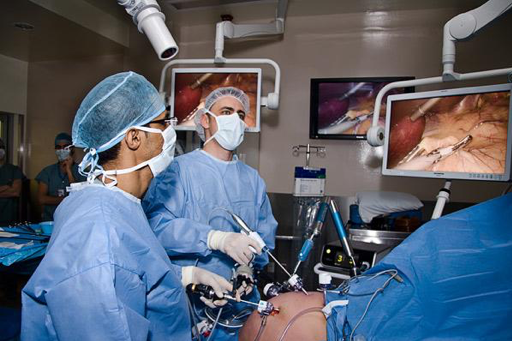
At any point the surgeon, resident, nurse, anesthetist or surgical tech can look down at the patient and see reactions, movement, bleeding outside of the camera view, breaks in sterility or reactions to medications administered, because the patient’s body is visible, not completely covered or surrounded by equipment while everyone is looking in a different direction than the patient is located.
The term “reverse adaptation” is when life changes its surroundings to suit its needs instead of adaptation being the organism changing to suit its surroundings. The hospitals are experiencing reverse adaptation. The Da Vinci Robot is an expensive initial purchase followed by a hefty maintenance contract and costly surgical tools. A hospital that had undertaken this project would undoubtedly want to see a return on investment with it. This means that even though there was no issue with patients seeking laparoscopic intervention for their medical needs, the hospital, and their staff may be “pushing” the patient to have the procedures done robotically.
Legally – “Intuitive is now facing at least 25 product liability lawsuits, and one attorney in the field confirmed that he personally has more than 1,000 potential clients who are claiming injuries from the device. While a Washington state jury cleared the company of liability last week in an initial case involving allegations that the company failed to properly train surgeons.”
If there is a surgical issue, the legal pathway has now been increased from the surgeon, hospital, and staff to also include the robot manufacturer. This becomes additional safety concerns for not just the equipment in the room, but the robot, software, service contracts, maintenance schedules, and required information associated.
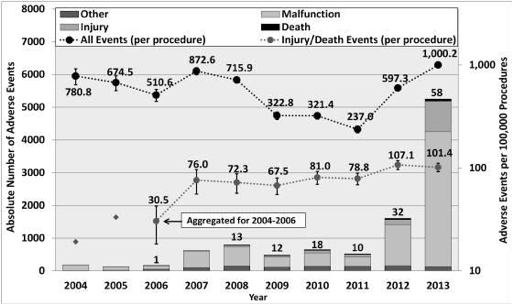
When the robotic systems were in their infancy and used sparingly there were fewer cases to study, but now with the increase in surgical services using the technology in various procedures, we can see the increases in injuries.
“Design input requirements were not adequately documented as required by 21 CFR 820.30(c). Specifically, you informed our investigator that you are aware of patient injuries associated with intraoperative cleaning of energized instruments such as the Monopolar Curved Scissors and Fenestrated Bipolar Scissors as evidenced by at least (b)(4) complaints and 82 MDRs during calendar years 2010 and 2011, and 15% of the MDRs reviewed by our investigator. You also informed our investigator that you are aware that cleaning instruments inside patients during surgery is a common practice and have included a label warning in the Instructions-for-Use (IFU) against the practice. When our investigator asked you to provide the design input documentation and design resolution of this known user need you failed to provide the requested documentation.”
Many of these lawsuits seem centered around the insulation of the “arms” carrying monopolar energy. Monopolar energy is very common during surgery; it is what we use to cauterize during procedures. There is a grounding pad that the current returns to the machine with and the instrument connected for delivery. In an open procedure, we can see the entire cautery working element. In laparoscopic, there are “blind spots” but we can see almost the entire element, the ports add to the shielding of current along with the insulation of the instrument shaft. In robotics, there is a very small field of view and not every part of the instrument can be visualized. Surgically we are all taught that a decision to make a deliberate action can only be done after obtaining what is known as a “critical view.” This was developed during cholecystectomies but holds true for all procedures. It is identifying structures, instrumentation, and space before taking a deliberate step such as cutting, stapling or cauterizing.
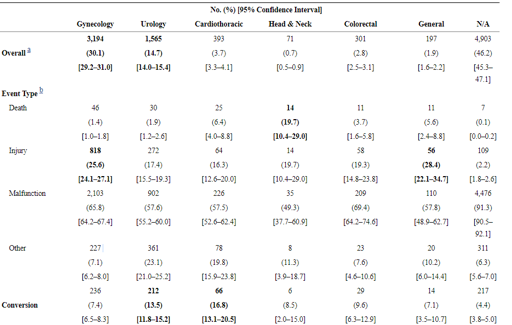
In this table, we can see the total amount of cases reported for each surgical service followed by how many deaths, injuries, and malfunctions for each. With cardiothoracic cases resulting in 20% death rate for complications and looking at seeing the gyn 3,194 robotic cases broken down by 46 deaths, 818 injuries, and 2,103 malfunctions, it warranted further investigation and reevaluation of the technology’s risk/reward benefit. The same report identified the most common technical issues as being:
“System errors and video/imaging problems contributed to 787 (7.4%) of the adverse events and were the major contributors to procedure interruptions, including system resets (274 cases, 82% of all system resets).”
“Falling of the broken/burnt pieces into the patient’s body constituted about 1,557 (14.7%) of the adverse events. In almost all these cases, the procedure was interrupted, and the surgical team spent some time searching for the missing pieces and retrieving them from the patient (in 119 cases, a patient injury, and in one case a death, was reported).”
“Electrical arcing, sparking, or charring of instruments and burns or holes developed in the tip cover accessories constituted 1,111 reports (10.5% of the events), leading to nearly 193 injuries, such as burning of tissues.”
“Unintended operation of instruments, such as uncontrolled movements and spontaneous powering on/off, happened in 1,078 of the adverse events (10.1%), including 52 injuries and 2 deaths.”
The report does break down the failing instrumentation question further and citing that approximately 62% were related to the device itself and 7% were caused by either staff or surgeon. Patient positioning was identified, however, my experience with robotic cases has the patients in steep Trendelenburg and very little opportunity for relieving stress on the pressure points. 15% was due to associated burns or electrosurgical arch during cutting or cauterization. This can be around the trocar ports where critical views are obstructed, or even if another instrument is in close proximity. One surgeon preference that does cause controversy is the modification to instruments. The protective coatings or insulation around the operative tip of the instruments have been known to have been “peeled back” providing a thinner tip for more of a fine approach.
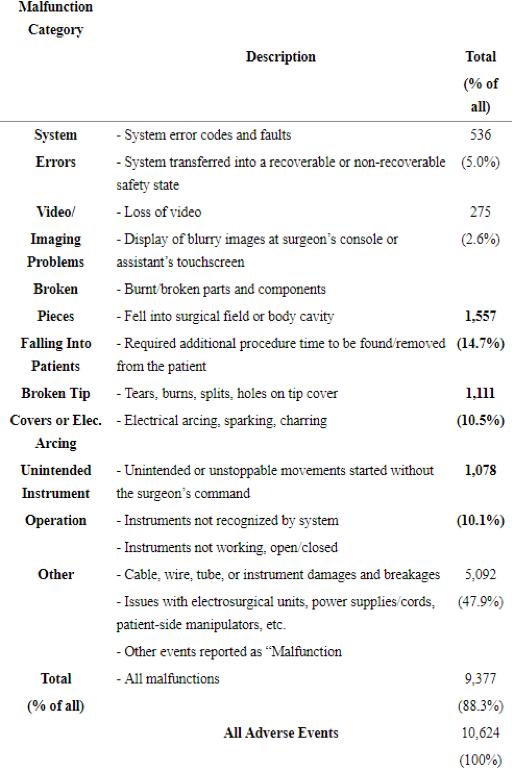
In the study on robotic gynecology titled: “Ten-Year Food and Drug Administration Reporting on Robotic Complications in Gynecologic Surgery,” Dr. Shields found 455 injuries and 177 malfunctions directly related to the robotic system itself and 50 product liability lawsuits. The FDA MAUDE report utilized shown that gyn cases totaled 61% of the robotic injuries with 81% leading to legal proceedings. The most frequent procedure reported was hysterectomy (n=399, 87%) and the complications were ureteral injuries (19%), infections (18%), and small-bowel injuries (16%). Ninety-three device-related deaths were reported citing sepsis (28%) and hemorrhage (18%).
Other legal concerns are with the length of surgery. If a prostatectomy can be done in 2 hours open but 4-5 hours robotically, the positioning associated could cause added harm. The arms may be tucked and unable to be assessed, this includes padding for scapula and shoulder braces that prevent patients from sliding in this position. The patient being in steep Trendelenburg also increases the intraocular pressure of the eyes causing postoperative blindness. Increased upper airway edema/resistance is seen in this position especially when combined with CO2 insufflation.
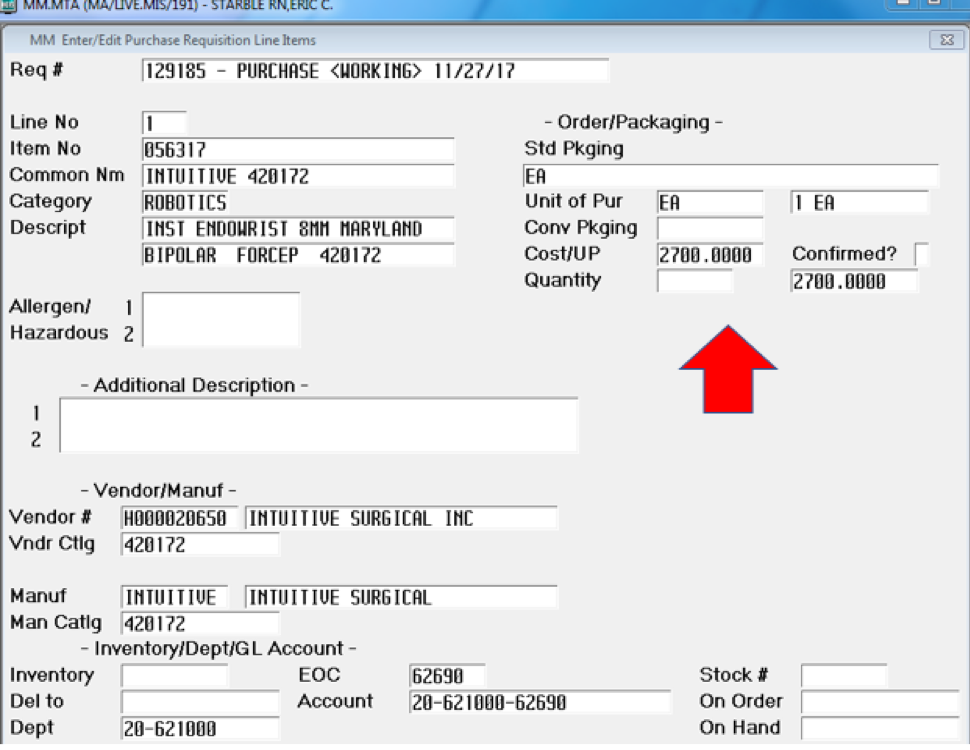
Wanting to show the drastic differences in price, I went under purchasing to find a couple of examples using the same instrumentation with one for robotic and one for laparoscopic. This is just the contract that we have at Mount Auburn Hospital, but through searches and research, I have found that all prices are similar and in those ranges. The purchase price that I am charged per unit in this example is $2,700 for one robotic Maryland style clamp on an Endowrist. This is a standard and frequently used laparoscopic instrument that is found in every kit pan (see Supplement A).
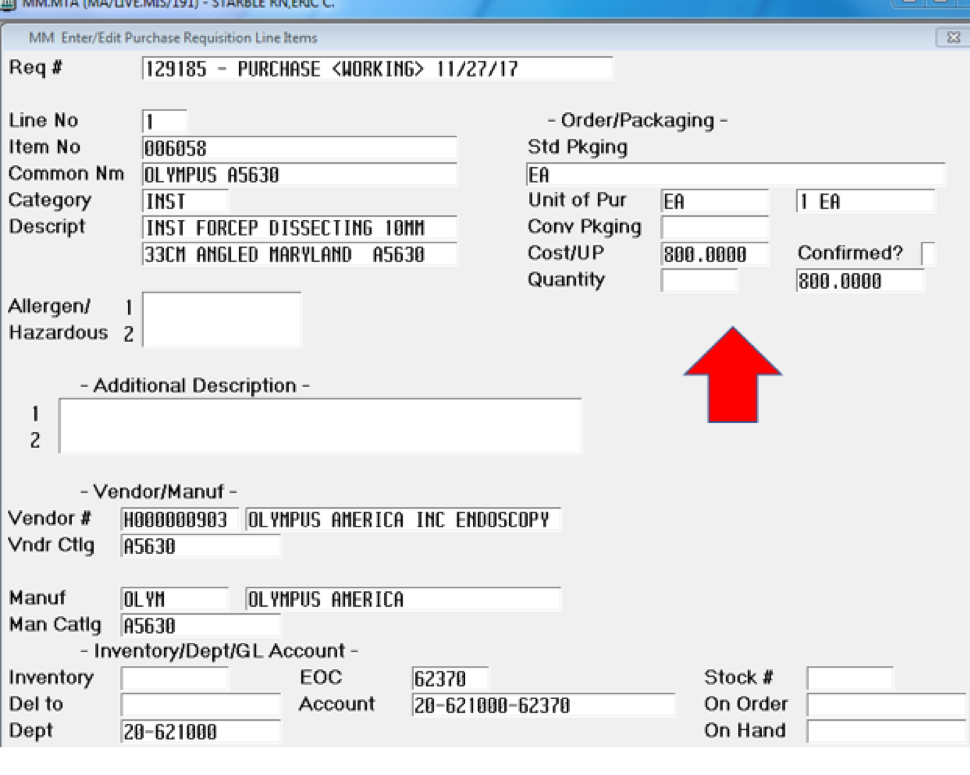
The same clamp style purchased for a standard laparoscopic instrument through Olympus is $800 (see Supplement B). There is a difference in size where one is 8mm and the other 10mm, which leads to the opportunity for smaller trocar ports. We do not use that technique; we try to make everything uniform with either 5mm or 10mm ports as a way to standardize costs and keep fewer variations of the same product.
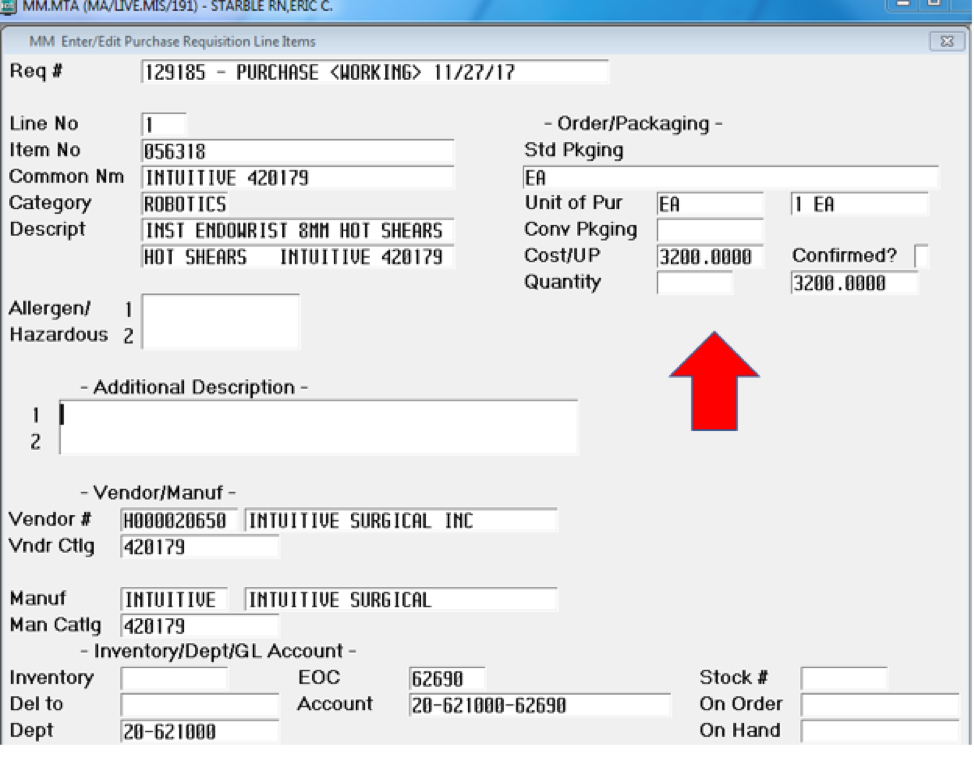
The Endowrist robotic shears or scissors are sold to us at a cost of $3,200 from Intuitive Surgical (see Supplement C). Each facility will negotiate their own contract; however, I am choosing the more commonly used instruments. Almost every laparoscopic case will use some form of scissor and dissecting device such as a Maryland. One of the few exceptions are appendectomies where once inside the abdominal cavity after insufflation, everything can be done with a grasper and two staple loads. Another example is laparoscopic inguinal hernia repair, where two graspers with dissecting until the defect is found and then a piece of polypropylene mesh is placed, occasionally using a tacker to hold until desufflation.
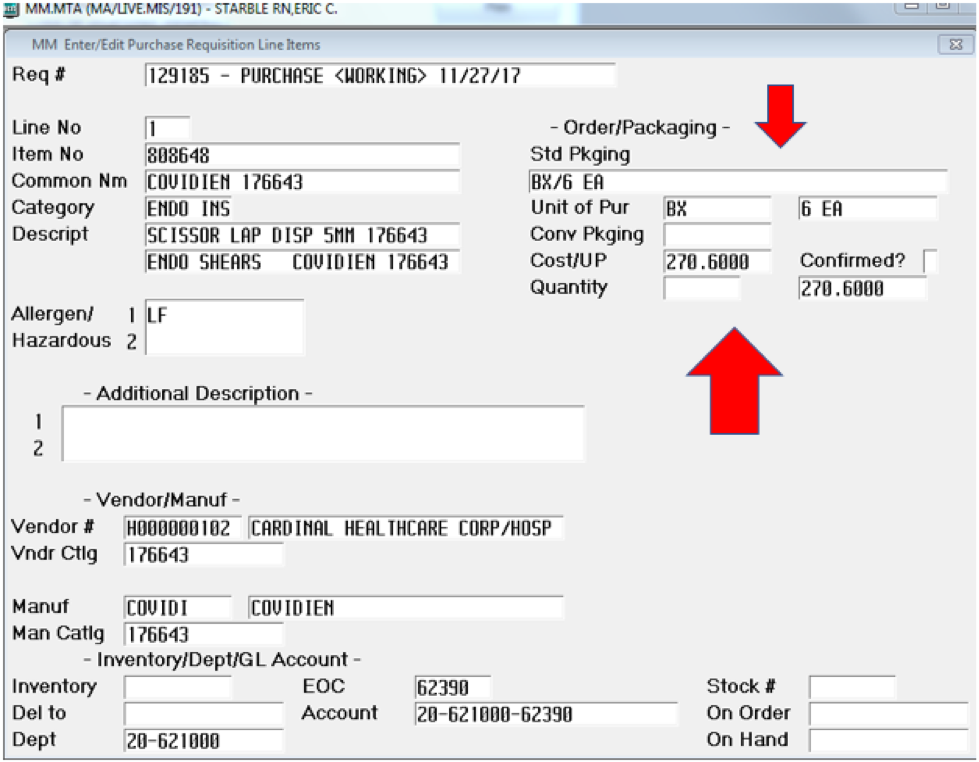
This I thought would be an interesting comparison. We purchase disposable laparoscopic shears/scissors from Covidien. Our contracted price is $270.60 for a box of 6 instruments. That makes our unit price $45.10 per instrument (see Supplement D). I will acknowledge but not go into the Stryker reprocessing of one-time-use instruments and the selling back of between hospitals since it is on a scale too small to add real value to the argument or economics. This makes the disposable laparoscopic shears 1-70.95th of the price. For each robotic scissor, we could have 71 disposable laparoscopic scissors.
The cost-based analysis for robotic intervention versus laparoscopic shows the amount of waste in the name of progress and innovation. One instrument for one Endowrist arm at a cost of $3,000 and can be used 10 times compared to an entire laparoscopy tray for $10,000 that has an estimated lifespan of 200 uses, is a fiscal upset. A typical laparoscopic set consists of approximately 10 instruments depending on facility preference and surgeon demands. If we were to purchase those same 10 instruments for robotics, it would be approximately $30,000. Now if we were to increase it to a level of 200 surgical cases, we are at $600,000 for the equivalent of a $10,000 purchase of laparoscopic instrumentation. Add the purchase price of $1.7, $2 or $2.3 million depending on estimates and an added service contract of $125,000 and we see a giant leap of faith taken by facilities to stay cutting edge. This translates into year one being anywhere from $2.5-$3 million dollars invested just to perform 200 laparoscopic cholecystectomies. The example of the robotic versus disposable laparoscopic shears applied to the 10-use lifespan now has another metric to contend with. The Endowrist has a 10-reprocessing turn lifespan, which means that it is disposed of before its 11th use and replaced with a new instrument. That makes our comparison for the scissor argument only apply to 10 procedural uses;
(10 x $45.10 = $451.00 < $3,200.00)
The need to recoup this investment is a defining factor for the “push” to have robotic usage in as many areas or fields as possible. We paid $1.8 million for the system, have a service contract of $150,000 annually and had to renovate an operating suite to accommodate the equipment and incidentals that are required to operate.
The current policy from Anthem Blue Cross Medicare Advantage states; “Anthem Medicare Advantage does not allow separate or additional reimbursement for the use of robotic surgical systems unless provider, state, federal, or CMS contracts and/or requirements indicate otherwise. Surgical techniques requiring the use of robotic surgical systems will be considered integral to surgical services and not a separate service. Reimbursement will be based on the payment for the primary surgical procedure(s).” This is one of the biggest determinants for advocating the use of robotic assistance. Unless the reimbursements increase, the faster and more inexpensive will remain the standard and therefore less experience for surgeons to learn robotic techniques ends with a longer path to efficiency of use.
The argument becomes “do the benefits to the hospital, surgeon and patient outweigh the costs financially and physiologically?” What is meant by this revolves around the price for purchase, maintenance, disposables, training, and housing the system evaluated against the advantages and disadvantages of increased cost to the patient and their insurers, surgical time, anesthetic time, surgeon skill-set, length of hospital stay, mortality rates, and outcomes.
In conclusion, my growing concerns over the use of new technology not being properly evaluated or vetted have not changed. The Da Vinci robot is a great tool for telesurgery on a battlefield, in space or deep sea where there may not be access to skilled surgical intervention. However, that is where it ends. The cost-based analysis for robotic intervention versus laparoscopic shows the amount of waste in the name of progress and innovation. The concerns for patient safety to be considered are in areas of informed consent. If the procedure that can be done as open, but is opted for laparoscopic for the smaller incision sites and less postoperative pain or recovery without any great increases financially, and it is the patient’s decision, their preferences should be followed. There are documented surgical studies providing reasoning for laparoscopic approach over open surgical technique and the patient benefit is well defined. The robotic approach has increased surgical times, increased risk and no added value over laparoscopy.
McNamee, D. (2014, August 1st). Are robots the future of surgery, or a pricey marketing gimmick? Retrieved from Medical News Today: https://www.medicalnewstoday.com/articles/280518.php?utm_source=TrendMD&utm_medium=cpc&utm_campaign=Medical_News_Today_TrendMD_1
Kang DC. (2010). Low quality of evidence for robot-assisted laparoscopic prostatectomy: results of a systematic review of the published literature. European urology, 930-937.
Jacobs, H. (2013, November 13th). Surgical robot da Vinci scrutinized by FDA after deaths, other surgical nightmares. Business Insider.
Trastulli, S. (July, 2015). Robotic versus Laparoscopic Approach in Colonic Resections for Cancer and Benign Diseases: Systematic Review and Meta-Analysis. PLOS ONE.
Lanfranco, A. R. (January, 2004). Robotic Surgery. annalsofsurgery, 14-21.
Surgical, I. (n.d.). https://www.intuitivesurgical.com/products/davinci_surgical_system/. Retrieved from Catalogue.
Sullins, J. P. (2014). Ethical trust in the context of robot-assisted surgery. The Society for the Study of Artificial Intelligence and Simulation of Behaviour. London, England: Sonoma State University.
AAGL. (March/April 2014). Guidelines for Privileging for Robotic-Assisted Gynecologic. Journal of Minimally Invasive Gynecology, Vol 21, No 2.
Bernstein, W. K. (2015). Anesthetic issues for robotic cardiac surgery. Annals of Cardiac Anesthesia, 58-68.
Alemzadeh, H. (April 20th 2016). Adverse Events in Robotic Surgery: A Retrospective Study of 14 Years of FDA Data. PLOS One.
Wright, J. D. (November 2014). Comparative Effectiveness of Robotically Assisted Compared With Laparoscopic Adnexal Surgery for Benign Gynecologic Disease. Obstetrics Gynecology, 886-896.
Breeden, J. T. (March 2013). Statement on Robotic Surgery by ACOG President James T. Breeden, MD. American Congress of Obstetrics and Gynecologists. Washington DC.
Tyler, J. A. (April 2013). Outcomes and costs associated with robotic colectomy in the minimally invasive era. Diseases of the Colon and Rectum, 458-66.
Griffen, M. (July 2013). The future of robotics: A dilemma for general surgeons. The American College of Surgeons.
Bankhead, C. (November 2011). Robot-assisted radical prostatectomy more costly than open surgery. Urology Times.
Finklestein, J. (Winter 2010). Open Versus Laparoscopic Versus Robot-Assisted Laparoscopic Prostatectomy: The European and US Experience. Reviews in Urology, 35-43.
Tang. (May, 2017). Robotic vs. Retropubic radical prostatectomy in prostate cancer: A systematic review and an meta-analysis update. Oncotarget.
Carlson, J. L. (May 2013). Medical boon or bust? Suits raise allegations of defects in da Vinci robot. Modern Healthcare.
Services, D. o. ( 7/16/13). Intuitive Surgical. San Francisco District: Food and Drug Administration.
Shields, K. (Nov 2015). Ten-year food and drug administration reporting of robot-assisted laparoscopy complications, deaths, and device malfunctions in gynecologic surgery. Journal of Gynecologic Surgery, 331-335.
Americas, O. C. (2012). Olympus Surgical Catalogue. Center Valley, Pennsylvania: Olympus Corporation.
Advantage, A. M. (2015, May 14th). Robotic Assisted Surgery. Anthem Medicare Advantage reimbursement policy.
Eric Starble is the Nurse at Mount Auburn Hospital based in Cambridge, Massachusetts.
HPHR.org was designed by ComputerAlly.com.
Visit HPHR’s publisher, the Boston Congress of Public Health (BCPH).
Email communications@bcph.org for more information.

Click below to make a tax-deductible donation supporting the educational initiatives of the Boston Congress of Public Health, publisher of HPHR Journal.![]()